Large validation study corroborates previous findings, sets the stage for clinical use
Researchers are one step closer to making a multi-cancer early detection (MCED) test, that can detect over 50 types of cancer, available to select candidates: those who are age 50 and older, asymptomatic, and considered high risk for the disease.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Findings from the third and final phase of the Circulating Cell-free Genome Atlas (CCGA) study have been published in the Annals of Oncology.
Study findings confirm that the test is proficient in detecting and classifying cell-free DNA (cfDNA), or tumor byproducts deposited in the bloodstream of a person with cancer. The test can also identify the site of the originating tumor, even in patients with no cancer-related symptoms.
Eric A. Klein, MD, first author of the paper and Chairman Emeritus of the Glickman Urological & Kidney Institute, says these findings corroborate those of a previous CCGA sub-study, but at a larger scale and with an independent validation set. He says these results set the stage for a new cancer screening paradigm.
“With the multi-cancer early detection tests, we have the opportunity to diagnose and treat cancer earlier. Used alongside other screening modalities, this could significantly reduce cancer-related deaths,” he says. For some high-mortality cancers – including liver, pancreatic and esophageal – this is the first screening test available.
Currently, only five cancer screening tests are available for patients in the United States; this includes tests for prostate, lung, breast, colorectal and cervical cancers. They each have limitations, including varying levels of invasiveness, discrepancies in use across clinical practice and high false-positive rates, which can lead to overdiagnosis and overtreatment.
The promise of this new assay is raising hopes that a new paradigm is afoot. It can detect the presence of circulating cfDNA through a single blood draw and is particularly effective when it comes to identifying more lethal and later-stage cancers, believed to have more cfDNA. However, this also underscores the importance of combining the MCED with existing screening tests until further refinements are made. “Prostate cancer, for example, sheds comparatively less DNA than other tumors, making it less likely to be detected by the novel assay,” explains Dr. Klein, a urologic oncologist.
How does it work? A genomic sequencing technology elucidates methylation, or chemical changes in the DNA that control gene expression, coupled with a machine learning application that systematically identifies patterns of irregularities in the DNA indicative of cancer. These patterns provide evidence as to where the cancer orginated and can help guide further diagnostic testing.
GRAIL, Inc. a California-based biotech company, developed the assay and has funded international research efforts. The MCED test is now available in the United States by prescription only.
The study evaluated the performance of the test in two cohorts: individuals already diagnosed with cancer (n = 2,823) and those without a cancer diagnosis (n = 1,254). It detected cancer signals from more than 50 types of cancer across all four stages of disease..
Dr. Klein says the team is satisfied with the promising findings; they are hopeful that this technology could be extrapolated as a tool for cancer screening at a population level.
In fall 2020, Cleveland Clinic announced that it would begin enrollment for its arm of the PATHFINDER study, of which Dr. Klein is the principal investigator. He explains that the strength of the CCGA study is its robust assessment of the assay itself. The PATHFINDER study, however, is intended to evaluate the care pathways from a cancer “signal detected” test in a primary care setting to arriving at a diagnostic resolution with a cancer specialist.
“We can say with confidence that the multi-cancer early detection test has clinical utility. We still don’t know the implications for its use in a more generalizable patient population, but the results are very promising.”
Disclosure: Dr. Klein is a consultant for GRAIL, Inc.

An underdiagnosed condition in patients with cancer
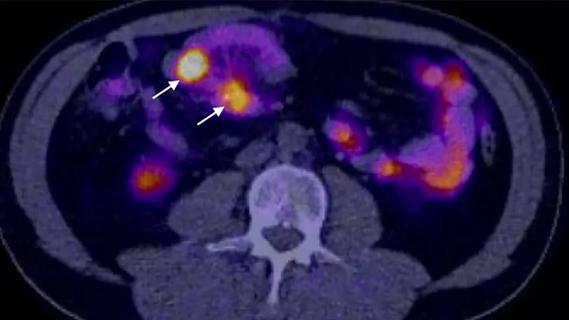
Study demonstrates superior visualization of occult primary lesions
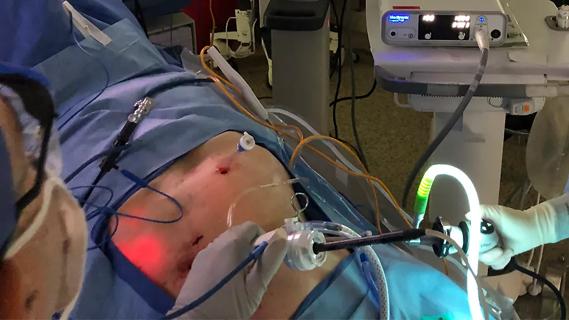
New device offers greater tumor control for malignant liver lesions
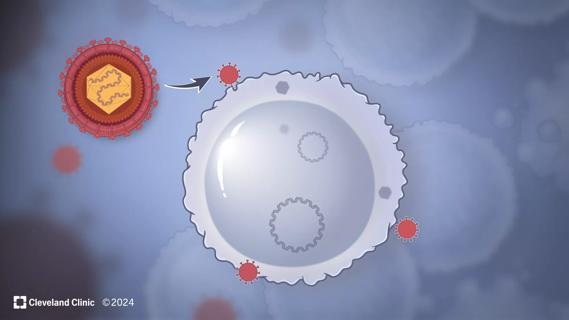
Cleveland Clinic researchers discover what drives – and what may halt – virus-induced cancer
First-ever U.S. population-level retrospective analysis reveals many patients with systemic mastocytosis need faster intervention

New program provides prehabilitation and rehabilitation services to help patients with cancer maintain and regain function

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses

Global R&D efforts expanding first-line and relapse therapy options for patients