Focus on evaluation and stepwise management are recommended in the treatment of acute agitation in pregnant women
by Joshua Niforatos, MTS, Jonathon Wanta, MD, Anna Shapiro, MD, Justin Yax, DO, DTMH, and Adele Viguera, MD, MPH
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Acute agitation in the pregnant patient should be treated as an obstetric emergency, as it jeopardizes the safety of the patient and fetus, as well as others in the emergency room. Uncontrolled agitation is associated with obstetric complications such as preterm delivery, placental abnormalities, postnatal death and spontaneous abortion.1
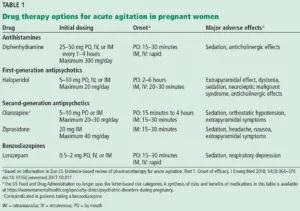
Current data on the reproductive safety of drugs commonly used to treat acute agitation—benzodiazepines, typical (first-generation) antipsychotics, atypical (second-generation) antipsychotics and diphenhydramine—suggest no increase in risk beyond the 2% to 3% risk of congenital malformations in the general population when used in the first trimester.2,3
Agitation is defined as the physical manifestation of internal distress, due to an underlying medical condition such as delirium or to a psychiatric condition such as acute intoxication or withdrawal, psychosis, mania or personality disorder.4
For the agitated pregnant woman who is not belligerent at presentation, triage should start with a basic assessment of airways, breathing and circulation, as well as vital signs and glucose level.5 A thorough medical history and a description of events leading to the presentation, obtained from the patient or the patient’s family or friends, are vital for narrowing the diagnosis and deciding treatment.
The initial evaluation should include consideration of delirium, trauma, intracranial hemorrhage, coagulopathy, thrombocytopenia, amniotic and venous thromboembolism, hypoxia and hypercapnia, and signs and symptoms of intoxication or withdrawal from substances such as alcohol, cocaine, phencyclidine, methamphetamine and substituted cathinones (“bath salts”). From 20 weeks of gestation to 6 weeks postpartum, eclampsia should also be considered in the differential diagnosis.1 Ruling out these conditions is important since the management of each differs vastly from the protocol for agitation secondary to psychosis, mania or delirium.
The US Food and Drug Administration (FDA) has discontinued its pregnancy category labeling system that used the letters A, B, C, D and X to convey reproductive and lactation safety. The new system, established under the FDA Pregnancy and Lactation Labeling Rule,6 provides descriptive, up-to-date explanations of risk, as well as previously absent context regarding baseline risk for major malformations in the general population to help with informed decision-making.7 This allows the healthcare provider to interpret the risk for an individual patient.
Reproductive safety of first-generation (i.e., typical) neuroleptics such as haloperidol is supported by extensive data accumulated over the past 50 years.2,3,8 No significant teratogenic effect has been documented with this drug class,7although a 1996 meta-analysis found a small increase in the relative risk of congenital malformations in offspring exposed to low-potency antipsychotics compared with those exposed to high-potency antipsychotics.2
In general, mid- and high-potency antipsychotics (e.g., haloperidol, perphenazine) are often recommended because they are less likely to have associated sedative or hypotensive effects than low-potency antipsychotics (e.g., chlorpromazine, perphenazine), which may be a significant consideration for a pregnant patient.2,8
There is a theoretical risk of neonatal extrapyramidal symptoms with exposure to first-generation antipsychotics in the third trimester, but the data to support this are from sparse case reports and small observational cohorts.9
Newer antipsychotics such as the second-generation antipsychotics, available since the mid-1990s, are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders.10 Recent data from large, prospective cohort studies investigating reproductive safety of these agents are reassuring, with no specific patterns of organ malformation.11,12
Recent studies of antihistamines, such as diphenhydramine, have not reported any risk of major malformations with first-trimester exposure to antihistamines.13,14 Dose-dependent anticholinergic adverse effects of antihistamines can induce or exacerbate delirium and agitation, although these effects are classically seen in elderly, nonpregnant patients.15 Thus, given the paucity of adverse effects and the low risk, diphenhydramine is considered safe to use in pregnancy.13
Benzodiazepines are not contraindicated for the treatment of acute agitation in pregnancy.16 Reproductive safety data from meta-analyses and large population-based cohort studies have found no evidence of increased risk of major malformations in neonates born to mothers on prescription benzodiazepines in the first trimester.17,18 While third-trimester exposure to benzodiazepines has been associated with “floppy-baby” syndrome and neonatal withdrawal syndrome,16 these are more likely to occur in women on long-term prescription benzodiazepine therapy. No study has yet assessed the risk of these outcomes with a 1-time acute exposure in the emergency department; however, the risk is likely minimal given the aforementioned data observed in women on long-term prescription benzodiazepine therapy.
If untreated, agitation in pregnancy is independently associated with outcomes that include premature delivery, low birth weight, growth retardation, postnatal death and spontaneous abortion.1 The risk of these outcomes greatly outweighs any potential risk from psychotropic medications during pregnancy.
Nevertheless, intervention should progress in a stepwise manner, starting with the least restrictive and progressing toward more restrictive interventions, including pharmacotherapy, use of a seclusion room, and physical restraints.4,19

Before medications are considered, attempts should be made to engage with and “de-escalate” the patient in a safe, nonstimulating environment.19 If this approach is not effective, the patient should be offered oral medications to help with her agitation. However, if the patient’s behavior continues to escalate, presenting a danger to herself or staff, the use of emergency medications is clearly indicated. Providers should succinctly inform the patient of the need for immediate intervention.
If the patient has had a good response in the past to one of these medications or is currently taking one as needed, the same medication should be offered. If the patient has never been treated for agitation, it is important to consider the presenting symptoms, differential diagnosis, as well as the route and rapidity of administration of medication. If the patient has experienced a fall or other trauma, confirming a viable fetal heart rate between 10 to 22 weeks of gestation with Doppler ultrasonography and obstetric consultation should be considered.
Mild to moderate agitation in pregnancy should be managed conservatively with diphenhydramine. Other options include a benzodiazepine, particularly lorazepam, if alcohol withdrawal is suspected. A second-generation antipsychotic, such as olanzapine in a rapidly dissolving form or ziprasidone, is another option if a rapid response is required.20
Severe agitation may require a combination of agents. A commonly used, safe regimen—colloquially called the “B52 bomb”—is haloperidol 5 mg, lorazepam 2 mg, and diphenhydramine 25 to 50 mg for prophylaxis of dystonia.20
The patient’s response should be monitored closely, as dosing may require modification as a result of pregnancy-related changes in drug distribution, metabolism and clearance.21
Although no study to our knowledge has assessed risk associated with one-time exposure to any of these classes of medications in pregnant women, the aforementioned data on long-term exposure provide reassurance that single exposure in emergency departments likely has little or no effect for the developing fetus.
Physical restraints along with emergency medications (i.e., chemical restraint) may be indicated when the patient poses a danger to herself or others. In some cases, both types of restraint may be required, whether in the emergency room or an inpatient setting.
However, during the second and third trimesters, physical restraints such as four-point restraints may predispose the patient to inferior vena cava compression syndrome and compromise placental blood flow.4 Therefore, pregnant patients after 20 weeks of gestation should be positioned in the left lateral decubitus position, with the right hip positioned 10 to 12 cm off the bed with pillows or blankets. And when restraints are used in pregnant patients, frequent checking of vital signs and physical assessment is needed to mitigate risks.4
This article was originally published here: Cleveland Clinic Journal of Medicine. 2019 April;86(4):243-247.

Consultation service provides comprehensive care to patients with anxiety, PTSD, schizophrenia, and other high-risk disorders

Study shows a growing openness to the clinical potential of psychedelic treatments

Urine test strips and point-of-care testing may be key to slowing opioid epidemic

Study sheds light on how clinicians addressed their patients’ pain and insomnia during the pandemic

Recovery's in Reach provides treatment options, peer support to those struggling with alcohol and drug use

Experts recommend task-focused strategy for monitoring treatment
Finding changes the treatment landscape of psychiatric illness

Patients benefit when doctors disclose with care