Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Apoptosis is suppressed by bi-allelic inactivation of the master regulator of apoptosis, p53, or its cofactor p16/CDKN2A, in about 80 percent of cancers. These genetic alterations impact therapy. Conventional oncotherapy applies stress upstream of p53 to upregulate it and cause apoptosis (cytotoxicity). When p53 is absent or nonfunctional, conventional therapy becomes toxic and futile.
Differentiation, on the other hand, cannot be completely suppressed since it is a continuum along which all cells exist. Neoplastic evolution stalls advances along this continuum at the most proliferative points — in lineage-committed progenitors, cells that intrinsically have division times measured in hours versus weeks or months for tissue stem cells. This differentiation arrest is by mutations/deletions to master differentiation-driving transcription factors or their coactivators, to shift balances towards corepressors that repress instead of activate hundreds of terminal-differentiation genes. In other words, malignant proliferation without differentiation, also referred to as cancer stem cell self-renewal, hinges on druggable corepressors. Inhibiting these corepressors (e.g., DNMT1) releases p53-independent terminal differentiation in cancer stem cells but preserves self-renewal of normal stem cells that express stem cell transcription factors.
DNMT1-depleting therapy’s selective effects on cancer stem cell self-replication and sparing of normal stem cells explain why 5-azacytidine and decitabine are the only two drugs approved for treatment of all myelodysplastic syndromes, including cases with complex cytogenetics and TP53 mutation. In this condition, it is critical to spare functionally normal stem cells needed to reverse low blood counts, the cause of morbidity and death.
Unfortunately, these observations in myeloid malignancies are not readily extended to p53/p16-null solid tumor malignancies, because decitabine and 5-azacytidine are inactivated within minutes by the pyrimidine metabolism enzyme cytidine deaminase (CDA) that is highly expressed in solid tissues. CDA upregulation within malignant cells is also a mechanism of resistance in myeloid malignancies.
Our research explores the combination of decitabine with a CDA-inhibitor (tetrahydrouridine) for orally administered, non-cytotoxic, DNMT1-depleting treatment of TP53-mutated solid and liquid cancers. This non-cytotoxic treatment also spares immune-effectors and increases cancer antigen presentation, constituting a logical platform for attempting to increase responses to immune checkpoint blockade.
Three clinical trials are currently under way. The first phase 2 trial assesses whether a combination of tetrahydrouridine-decitabine (THU-Dec) and nivolumab is more effective than nivolumab monotherapy in patients with non-small cell lung cancer. Our primary outcome is objective response rate by RECIST1.1. We will also measure progression-free survival and overall survival.
We are also currently recruiting participants with refractory/relapsed lymphoid malignancies for a phase 1, single-arm trial testing the efficacy of THU-Dec in these patients. Finally, we are recruiting patients with advanced pancreatic cancer that has progressed through one or more lines of therapy for a third, phase 1 trial testing the efficacy of THU-Dec.
For more information about these trials, or to refer your patients, visit clevelandclinic.org/cancerclinicaltrials
Dr. Saunthararajah is staff in the Department of Hematologic Oncology and Blood Disorders, Professor of Medicine at Cleveland Clinic Lerner College of Medicine, and co-leads Case Comprehensive Cancer Center’s Developmental Therapeutics Program.

First-of-its-kind research investigates the viability of standard screening to reduce the burden of late-stage cancer diagnoses
Study demonstrates ability to reduce patients’ reliance on phlebotomies to stabilize hematocrit levels

Findings highlight an association between obesity and an increased incidence of moderate-severe disease
Cleveland Clinic Cancer Institute takes multi-faceted approach to increasing clinical trial access
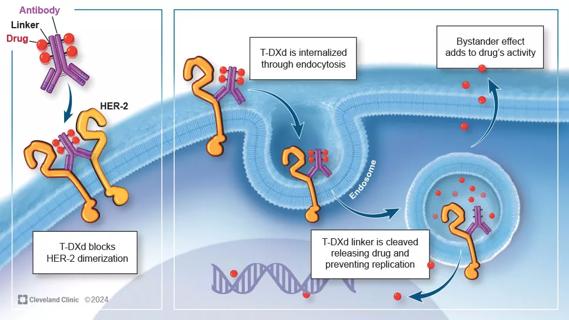
Key learnings from DESTINY trials

Gene editing technology offers promise for treating multiple myeloma and other hematologic malignancies, as well as solid tumors

Study of 401,576 patients reveals differences in cancer burdens as well as overall survival

Enfortumab plus pembrolizumab reduced risk of death by 53% compared with platinum-based chemotherapy