Huge postmarketing study also finds no increase in complication rate

Placement of cardiac implantable electronic devices (CIEDs) inside an absorbable, antibiotic-eluting envelope reduced the incidence of major infections by 40 percent relative to standard-of-care CIED infection prevention strategies alone, according to results of the international WRAP-IT randomized controlled trial.
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Rates of CIED infection are generally low, but when these infections occur, they can be devastating for patients, resulting in prolonged hospitalization, removal of the device system, possible vascular injuries and sometimes fatality,” says Cleveland Clinic electrophysiologist Khaldoun Tarakji, MD, MPH, global principal investigator of WRAP-IT, who reported the results in a late-breaking trials presentation at the American College of Cardiology (ACC) scientific session on March 17. The study was published simultaneously in the New England Journal of Medicine. “These findings provide strong evidence for use of the envelope to reduce CIED infection risk.”
This evidence may expand adoption of the envelope (TYRX™ Absorbable Antibacterial Envelope, Medtronic), which was approved by the FDA in 2013. The product was designed to surround a CIED implanted in the chest. It consists of a knitted mesh body coated with an absorbable polymer mixed with the antibiotics minocycline and rifampin, which are eluted into local tissue for at least seven days. The envelope is absorbed by the body within about nine weeks.
“Evidence on prophylactic strategies to reduce CIED infection beyond the use of preoperative antibiotics has been limited,” notes Cleveland Clinic electrophysiologist Bruce Wilkoff, MD, who served as the WRAP-IT trial’s steering committee chair. “Several studies have yielded supportive evidence for local antibiotic delivery, but a large randomized trial has been lacking.”
WRAP-IT was launched to help address that deficiency through its size and design. The study randomly assigned 6,983 patients from 25 nations to receive the envelope or not receive the envelope in addition to standard-of-care infection prevention (pre-procedure intravenous antibiotics plus sterile technique) during their CIED procedure. Randomization was done on a 1:1 basis with patients stratified by device type, which included pacemaker/cardiac resynchronization therapy (CRT) pacemaker or implantable cardioverter defibrillator/CRT defibrillator. Patients (mean age, 70.1 years) were blinded to their treatment group assignment, and the groups were well matched for baseline and procedural characteristics.
“Our enrollment strategy was to include individuals at increased risk for CIED infection based on literature evidence,” notes Dr. Tarakji. This included those undergoing CIED generator replacement or system upgrade with or without new leads, as well as de novo CRT defibrillator implantation or pocket or lead revisions.
“Management of patients with a CIED involves more than just the implantation — there are follow-up procedures for upgrades, battery or lead changes, and the like,” Dr. Wilkoff adds. “Every time the CIED pocket is revisited, there is a risk of infection.”
The primary endpoint was the incidence of major CIED infection within 12 months of the procedure, with major infections defined as those requiring system extraction or revision, requiring long-term antibiotic therapy with infection recurrence, or resulting in death. A secondary safety endpoint was procedure- or system-related complications within 12 months.
Major CIED infections occurred in 25 patients in the envelope group compared with 42 patients in the control group, for 12-month Kaplan-Meier event rate estimates of 0.7 percent and 1.2 percent, respectively (hazard ratio [HR] = 0.60; 95% CI, 0.36-0.98; P = 0.041), representing a 40 percent relative reduction in the risk of developing a major CIED infection.
On the safety front, procedure- or system-related complications occurred in 201 patients in the envelope group and 236 patients in the control group, for 12-month Kaplan-Meier event rate estimates of 6.0 percent and 6.9 percent, respectively (HR = 0.87; 95% CI, 0.72-1.06; P < 0.001 for noninferiority).
Mean follow-up was 20.7 months, with 89.4 percent of enrollees completing at least 12 months of follow-up. Across the entire follow-up period, rates of major CIED infections were again significantly lower in the envelope group than in the control group, with 36-month Kaplan-Meier event rate estimates of 0.9 percent and 1.6 percent, respectively (HR = 0.63; 95% CI, 0.40-0.98).
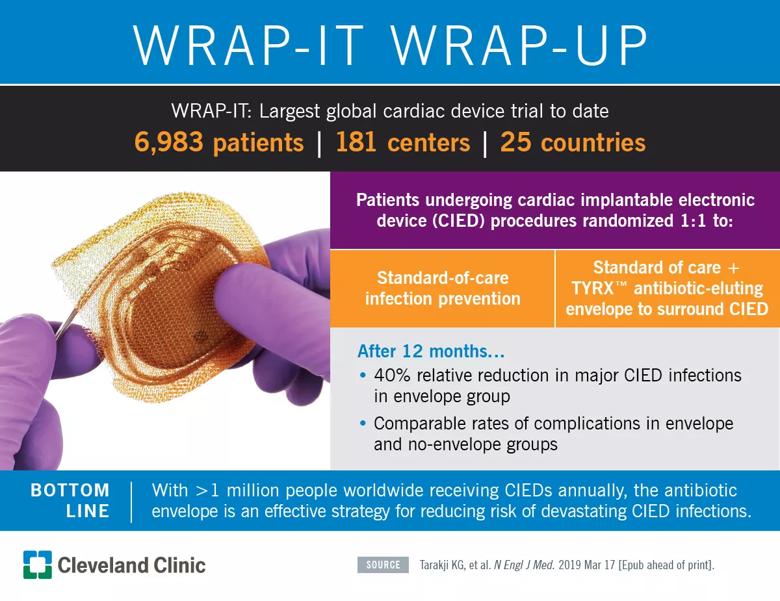
“In a population at increased risk of CIED pocket infection, the antibiotic envelope was significantly more effective at preventing infection than standard infection control strategies by themselves,” observes Dr. Tarakji. “The envelope was safely implanted in 99.7 percent of procedure attempts, and it did not increase complications despite the fact that use of the envelope may require dissection of a slightly larger CIED pocket.”
The researchers called attention to two additional notable findings from the study:
“This study, which is the largest global cardiac device trial ever completed, provides a realistic look at the number of device infections associated with CIEDs,” says Dr. Wilkoff. “While overall infection rates were low, the envelope still provided significant benefit to patients. That’s important in view of the fact that well over 1 million patients receive CIEDs worldwide every year.”
The study was supported by Medtronic. In addition to being presented as a late-breaking trial at the ACC meeting on March 17, the study is being presented by Dr. Wilkoff during a late-breaking trials session at the European Heart Rhythm Association Congress on March 18.
Image of TYRX envelope at top courtesy of Medtronic.
Surprise findings argue for caution about testosterone use in men at risk for fracture

Findings support emphasis on markers of frailty related to, but not dependent on, age
![GettyImages-1252287413 [Converted]](https://assets.clevelandclinic.org/transform/StoryPanel/350804b2-f1e4-4d97-a277-9629cf45af3e/23-HVI-4120348_redlining_650x450_jpg?w=3840&q=75)
Large database study reveals lingering health consequences of decades-old discrimination
Additional analyses of the two trials presented at 2023 ESC Congress
Prospective SPIRIT-HCM trial demonstrates broad gains over 12-month follow-up

An ACC committee issues recommendations to accelerate sluggish progress
Review of our recent experience shows it’s still a safe option
Machine learning may improve risk prediction and guide therapy