Risk factors for antidepressant discontinuation syndrome, and guidance for its management

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/16aa729c-12d9-402f-9fa1-cec961f8b5ea/antibiotics-and-myasthenia-gravis-exacerbations-1130531561)
Pain medications
By Samantha J. Zwiebel, MD, and Adele C. Viguera, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This article is reprinted from the January 2022 issue of the Cleveland Clinic Journal of Medicine (2022;89[1]:18-26).
An ever-increasing number of patients are prescribed antidepressant medications, most often by primary care physicians,1 with approximately 12.7% of the adult U.S. population prescribed a daily antidepressant.2 Antidepressants are used to treat a variety of conditions other than mood and anxiety disorders, such as chronic pain syndromes, tobacco use disorder and obsessive-compulsive disorder. Common scenarios prompting discontinuation of an antidepressant include the following:
In the case of insurance coverage, clinicians are encouraged to advocate for the patient whenever a coverage issue arises in order to avoid changing antidepressants. Additionally, patients may choose to stop an antidepressant of their own accord.
Stopping antidepressants can be challenging because of the frequency of antidepressant discontinuation syndrome (ADS). Discontinuation symptoms occur commonly and vary in severity.5 Symptoms will often surprise and frighten patients who are not forewarned, leading them to seek emergency medical care. Discontinuation symptoms include insomnia, flu-like symptoms, mood disturbances, dizziness, paresthesias (“brain zaps”) and a broad array of other adverse effects.6,7
Advertisement
The range of risk factors for ADS indicates the complex mechanisms underlying discontinuation symptoms beyond acute medication cessation. But despite the frequent morbidity associated with stopping antidepressants, there is a notable lack of guidance for clinicians on tapering antidepressants or managing ADS,8 and the majority of available guidelines are not considered evidence-based.5 The following narrative review describes the array of discontinuation symptoms and their causes and provides practical clinical guidance for discontinuing antidepressants to minimize the risk of ADS. The principles discussed regarding medication discontinuation and switching to another antidepressant can be applied regardless of the antidepressant indication.
ADS can pose diagnostic challenges because many symptoms of discontinuation overlap with those of toxicity and recurrence. ADS should be suspected if the patient reports either new symptoms that were not part of the original presentation or symptoms noted in the original presentation but at greater severity.9
ADS can be conceptualized into three categories:
By contrast, new episodes of the original condition can occur after discontinuation, including relapse (same symptoms at the same severity emerge < 6 weeks) and recurrence (same symptoms at the same severity emerge > 6 months).9 These definitions are summarized in Table 1.9
Advertisement
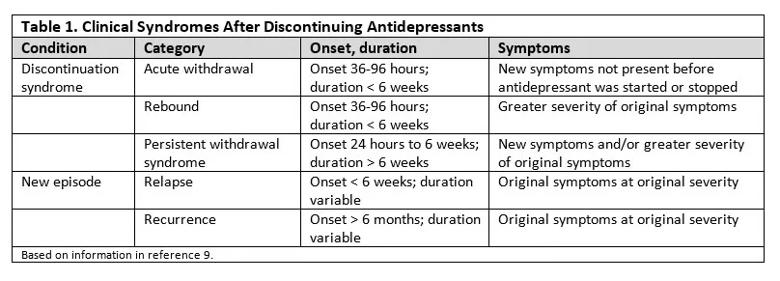
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/73ae61d4-7116-4372-a696-d972f7cc1386/22-NEU-2892119-CQD-Inset-Table1_jpg)
Risk of relapse. Patients who stop antidepressants, regardless of the specific psychiatric indication or duration of taper, are at a greater risk of relapse of psychiatric illness than patients who remain on antidepressants.10 A large meta-analysis10 found the rate of 12-month relapse was 2.3 times higher in patients who had stopped antidepressants compared with those who continued treatment. Of patients who stopped antidepressants, 44.8% experienced a relapse or recurrence within 12 months compared with 19.5% of patients who continued antidepressants. The patients who stopped antidepressants also demonstrated a median time to recurrence of about 14 months, compared with 48 months for patients who continued antidepressants.10 Therefore, it is especially critical to distinguish between a recurrent episode and ADS.
Studies have shown that 27% to 86% of patients who attempt to stop antidepressants, whether on their own or under supervision of a physician, experience ADS.11 One review11 included studies ranging from randomized clinical trials to online surveys, with a weighted average of 56%. Of patients experiencing discontinuation symptoms, 86.7% reported ongoing symptoms at two months, 58.6% at one year and 16.2% beyond three years.11 Persistent discontinuation symptoms, often termed protracted withdrawal syndrome, is not formally defined but should be diagnosed when discontinuation symptoms persist beyond several months. Data from an Internet forum for patients12 identified a mean duration of 37 months and a median duration of 26 months in persistent symptoms. Of forum users identified as having protracted withdrawal, 73.9% experienced various physical symptoms, 82.6% experienced affective symptoms and 63.8% experienced both physical and affective symptoms. Sleep disturbances (43.5%) and cognitive symptoms (31.9%) were also very common.12
Advertisement
Symptoms vary by drug class. Symptoms of ADS present across a range of organ systems and vary based on the antidepressant class and individual medication. Table 2 summarizes the most typical symptoms, though symptoms can vary by individual medication since many medications, even within the same class, have different receptor profiles and affinities.6,7,13,14 There is relatively little information available on tricyclics, tetracylics and monoamine oxidase inhibitors (MAOIs) relative to selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), in part because symptoms were often thought to be psychosomatic13 and not of much importance at a time when tricyclics, tetracyclics and MAOIs were more commonly used.9
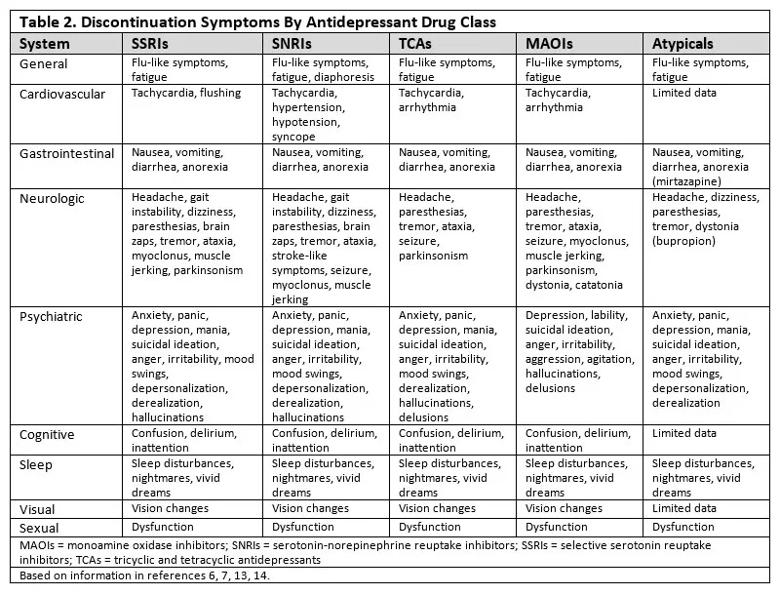
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/44a4367e-870d-489e-b27d-dd0001db96db/22-NEU-2892119-CQD-Inset-Table2_jpg)
There are also only limited data on atypical antidepressants such as mirtazapine and bupropion and newer medications such as vortioxetine and levomilnacipran.
A useful diagnostic mnemonic. To simplify recognition of ADS, clinicians can use the mnemonic FINISH for flu-like symptoms, insomnia, nausea, imbalance (dizziness), sensory disturbances (paresthesias, including brain zaps) and hyperarousal (anxiety, irritability),15 which are fairly generalizable across antidepressant classes.
Suicidal ideation, panic and other risks. Discontinuation of antidepressants can carry significant risk, including suicidal ideation, suicide and mania. One study16 of 73 patients investigating the role of adjunctive psychotherapy in antidepressant discontinuation had to be stopped due to ethical concerns stemming from lack of efficacy and a patient suicide. Another study17 observed 28 patients undergoing antidepressant discontinuation and found suicidal ideation in four patients, all of whom were stopping paroxetine.
Advertisement
Panic and restlessness due to ADS likely exacerbate suicidal ideation, which in most cases is probably due to ADS rather than relapse.6 Patients treated for panic disorder should be closely monitored for rebound.
Cessation of antidepressants can also elicit mania or hypomania. Although it is more frequent that patients experience depression following antidepressant discontinuation, the phenomenon of mania due to antidepressant cessation has been observed in at least 24 cases across various antidepressants.18
Brain zaps: Unpleasant, sometimes disabling. Another notable discontinuation symptom is a type of paresthesia known colloquially as “brain zaps,” unpleasant and sometimes disabling electric shock-like sensations. These sensations may be due to adrenergic withdrawal. Therefore, SNRIs are expected to have a higher likelihood of causing brain zaps than SSRIs. Disproportionately higher rates of brain zaps are reported with venlafaxine but also with paroxetine, and they have been reported with many antidepressants.19
Electric shock-like sensations are most often seen in patients who have abruptly stopped antidepressant treatment or missed doses, though more than 30% of patients with brain zaps report these symptoms either while undergoing a taper or after completing a taper. Some patients may experience the zaps during normal treatment.
The sensations typically last a few weeks but can persist for years and may be associated with dizziness, the perception of crackling or sizzling noises, and various other symptoms such as depersonalization (feeling detached from and outside of one’s own body) and derealization (the sensation that the external world is unreal). Common triggers are eye and head movements, but patients note many other triggering activities. There are no known treatments that successfully and specifically target brain zaps other than resuming the medication at the previous dose.19
There is controversy surrounding the terminology of ADS versus antidepressant withdrawal. While clinically the terms are often used interchangeably, it is important to appreciate the nuanced meaning of each term.
Withdrawal from antidepressants may imply addiction.3,20 However, patients do not crave antidepressants, experience intoxication or exhibit other hallmarks of a substance use disorder, such as using more and more drug despite negative consequences.17 Therefore, clinically, the term discontinuation is preferred over withdrawal. Some other authors argue that the term discontinuation downplays the severity of the emergent symptoms, which can be intensely uncomfortable and impairing.21 These authors strongly favor the use of antidepressant withdrawal because the syndrome of antidepressant discontinuation is consistent with withdrawal from other substances,6,7,21,22 and because antidepressants cause dependence.12
ADS has been clinically recognized for decades,13,23-25 demonstrating emergence of symptoms within days or even hours of abrupt cessation. Clinical trials that transitioned patients on antidepressants to placebo observed discontinuation symptoms, especially in antidepressants with shorter half-lives.23,24 However, many patients experiencing such symptoms are misdiagnosed as experiencing a relapse or a psychosomatic reaction.13,25 With growing recognition of the difficulty in stopping antidepressants, the risk of ADS should be discussed with patients as part of an informed-consent process when starting antidepressants.25
Straightforward pharmacokinetic explanations of discontinuation symptoms are inadequate since symptoms continue to emerge over a period of weeks and can last for months and even years.22 Antidepressants are believed to exert their effect partly through chronic medication exposure leading to receptor downregulation to create a more favorable neurotransmitter-to-receptor ratio.6,7,26-28 It should be noted that each class of antidepressants exerts its effect in different ways; therefore, broad statements about antidepressant mechanisms of action tend to be overgeneralizations. Still, the mechanism of receptor downregulation, which requires weeks to months and may over time contribute to medication desensitization, likely accounts in part for prolonged discontinuation symptoms.12
Furthermore, it is also hypothesized that antidepressants with anticholinergic properties may lead to receptor upregulation rather than downregulation and thus can contribute to desensitization.13 The theory of “oppositional tolerance” incorporates both upregulation and downregulation,22 because discontinuing antidepressants may lead to a variety of rebound symptoms due to the widespread distribution of serotonin throughout the body. Oppositional tolerance that develops and increases over time may explain why patients treated with antidepressants at higher doses and for longer periods are at greater risk for ADS.29
Despite extensive literature on ADS, there is still little known about the patient characteristics that pose the most risk.7,14 Nevertheless, though the risk of ADS cannot be eliminated, it can be reduced through awareness of known risk factors (Table 3).6,7,14,23,24,26,28-32
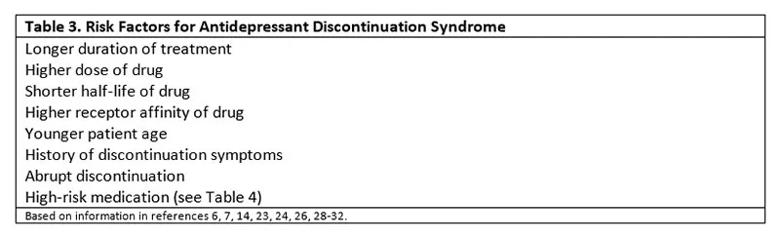
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/43296267-255a-4943-83f8-835fd7ccccb2/22-NEU-2892119-CQD-Inset-Table3_jpg)
Some patients may have a genetic predisposition,7 although this is yet to be determined. Younger patients may be more at risk, but younger patients are also more likely to abruptly discontinue their antidepressants.30 Patients who report discontinuation symptoms during missed doses are also at higher risk of withdrawal during a taper,28 and patients should be asked about this experience during an appointment to plan a taper.31
Treatment of longer duration6,26,29 and treatment at higher doses29 have proven to be risk factors for ADS. Medications with higher receptor affinity such as paroxetine carry greater risk.26 Abrupt discontinuation of antidepressants increases the risk of ADS.32 The indication for the prescribed antidepressant does not appear to affect the likelihood of ADS symptoms.6
Medication half-life inversely predicts the risk of discontinuation.6,23,24 Of the SSRIs, paroxetine and fluvoxamine have the shortest half-lives and therefore are associated with the highest risk. Citalopram, escitalopram and sertraline carry moderate risk, while fluoxetine carries the lowest risk due to the long half-life of its active metabolite (approximately 7 days).7 Among SNRIs, venlafaxine and desvenlafaxine carry the highest risk for ADS. Duloxetine carries a high risk and milnacipran a low risk, with levomilnacipran carrying the lowest risk.14 MAOIs and tricyclic and tetracyclic antidepressants generally carry a relatively high risk for ADS. Although mirtazapine and bupropion carry some risk, the data on this are limited.6
To date, there are only minimal prospective data comparing the risk of ADS with different antidepressants. A recent review9 concluded that paroxetine and venlafaxine pose the greatest risk among antidepressants most commonly prescribed today. Table 4 stratifies antidepressants by the risk of ADS.
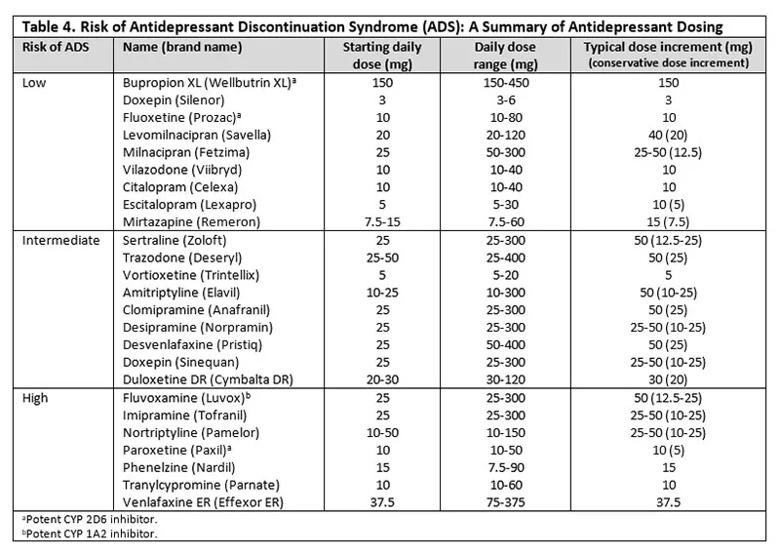
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/63df1dc3-f837-4651-b393-80cde1b15d8b/22-NEU-2892119-CQD-Inset-Table4_jpg)
To date, no formal schedules for tapering antidepressants have been validated. The maxim “slower is better” applies to tapering antidepressants. Most authors now agree that a longer taper, defined as at least 14 days, may reduce the risk of discontinuation symptoms compared with a rapid taper, defined as 1 to 10 days.17 Current recommendations advise antidepressant dose adjustments every 1 to 4 weeks.26 Data from the field of sleep medicine, where patients are often instructed to hold antidepressants, suggest tapers over 3 to 4 months.33 Some have suggested the “10% rule,” which recommends reducing the dose by 10% weekly.26 Switching to a liquid formulation can facilitate slow tapers by enabling precise dosing. However, neither paroxetine nor venlafaxine, common antidepressants very likely to cause ADS, is readily available in liquid formulation. Patients report opening capsules to divide up beads, as well as breaking unscored tablets with pill cutters to slowly taper off medication.11,28 Patients can also use compounding pharmacies to obtain custom formulations.
When switching from one antidepressant to another, taper considerations are different. Cross-tapering is generally recommended4 and can be carried out over 1 to 4 weeks or longer depending on the dosing of the original medication. Cross-tapering involves incrementally decreasing the current antidepressant while incrementally increasing the new antidepressant (Table 4).
Other ways to change antidepressants include starting the new antidepressant immediately after stopping the previous drug (direct switch), starting the new drug after 2 to 3 days off the prior drug (moderate switch), and starting the new drug after five half-lives off the prior drug (conservative switch). A direct switch can be considered when changing an SSRI to another SSRI or SNRI at an equivalent dose, unless the current SSRI is at a high dose. Moderate and conservative switches carry the risk of ADS due to the time spent completely off an antidepressant.4 Alternatively, cross-tapering carries the risk of causing drug-drug interactions, and clinicians should be aware of potential risks such as serotonin syndrome. None of these switching methods is appropriate for MAOIs, which require a 14-day washout both pre- and post-treatment, and at least a 5-week washout if MAOI treatment is preceded by fluoxetine.34
Studies have suggested discontinuing antidepressants by switching patients to fluoxetine due to its 7-day half-life and relatively low risk of ADS.23,35 There are no formal guidelines on fluoxetine-assisted tapers, but the strategy consists of cross-tapering to fluoxetine (slowly decreasing the original antidepressant while increasing fluoxetine to a dosing required for ADS remission) followed by discontinuation of fluoxetine,26 which can self-taper due to its long half-life. Such a strategy can be helpful when tapering off venlafaxine, desvenlafaxine, paroxetine, and tricyclic and tetracyclic antidepressants. However, clinicians must be wary of drug-drug interactions due to fluoxetine’s CYP 2D6 inhibition, which can raise drug levels.
Additional practical guidance on discontinuing and switching antidepressants can be found online.36,37
Patient education and other management tools. Perhaps the most pragmatic and effective measure in managing ADS is to proactively educate patients about FINISH symptoms with missed doses or abrupt discontinuation. This prepares patients to better cope with ADS during planned antidepressant discontinuation and may prevent unnecessary visits to the emergency room. It is also important to reassure the patient that ADS is neither life-threatening nor indicative of addiction, and that it will eventually resolve.
If ADS does occur, the antidepressant being tapered can be rapidly resumed at the previous dosing and then tapered more slowly. This intervention is the simplest antidote and is highly effective in most cases within 24 hours.
Symptom management is another treatment strategy.35 For example, headaches can be managed with ibuprofen 400 mg or acetaminophen 650 mg every 4 to 6 hours. Nausea can be addressed with ondansetron 4 mg every 8 hours. Anxiety and insomnia can be managed with hydroxyzine 50 mg every 6 hours or with benzodiazepines. In some cases, diphenhydramine can be recommended if the patient is stopping a particularly anticholinergic antidepressant, such as paroxetine.
Additionally, antidepressants can have extensive drug-drug interactions. These interactions can occur from enzymatic induction or inhibition of the cytochrome P450 system or from medication side effects that overlap with the effect of other medications, such as additive effects in anticoagulation. The cessation of an antidepressant therefore requires the reevaluation of other concurrently prescribed medications.22 While it is always recommended to complete a medication review, changes in bupropion, fluoxetine and paroxetine (potent CYP 2D6 inhibitors), fluvoxamine (a potent CYP 1A2 inhibitor), tricyclic and tetracyclic antidepressants, and MAOIs should prompt an especially close medication review.
Translational efforts, including the further development of animal models for ADS, are needed to enhance our understanding of the pathogenesis, phenotypic variance and role of genetic and environmental modulation in ADS. Such efforts will help the clinician screen for a medication’s potential to induce ADS and will contribute to developing agents that reduce the symptoms and severity of ADS.38
Dr. Zwiebel, a former fellow in consultation-liaison psychiatry at Cleveland Clinic, is Assistant Professor of Clinical Psychiatry, Department of Psychiatry, University of Pennsylvania. Dr. Viguera is a staff psychiatrist in the Department of Psychiatry and Psychology, Cleveland Clinic.
Advertisement
New study highlights the need for plain-language prompting and human oversight in addiction care communication
What hospital clinicians get right — and wrong — when diagnosing depression and delirium
Swift, aggressive thiamin therapy may be key to preventing long-term neurological injury
Case underscores potential dangers of combining psychedelics with tranylcypromine and stimulant medications
Researchers explore how changes in the gut microbiome influence the brain's reward response to alcohol
Researchers explore new avenues for the management of psychiatric illness in patients with seizure disorders
Addiction experts use decades of research and clinical experience to help patients overcome substance use disorders
Specialized course helps APPs navigate clinical concerns and interpersonal skills