Diagnosis and management tips
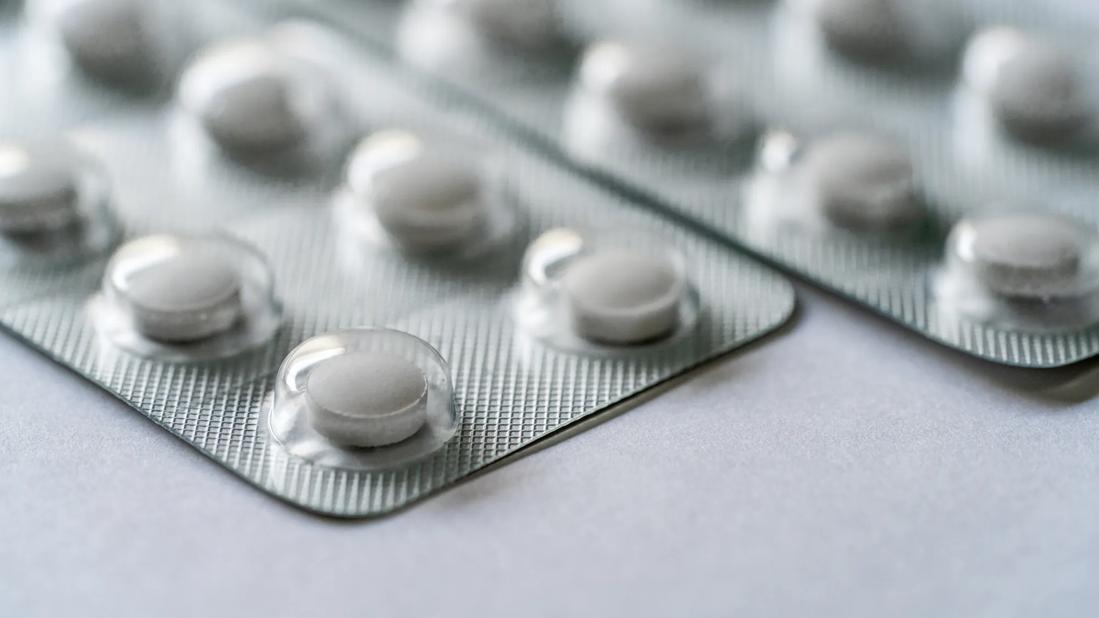
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/acc46bf8-9957-48c1-b792-94da562c1a60/Gastroparesis-nongastroenterologist-Medications)
Medication
Note: This article is reprinted from the Cleveland Clinic Journal of Medicine (2024;91[6]:373-383). The first portion of the published journal article provided an overview of the condition and its primary symptoms. Part II focuses on the diagnosis and management of gastroparesis.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Written by Jorge Araujo-Duran, MD, Arjun Chatterjee, MD, and Samita Garg, MD
Gastroparesis is a chronic motility disorder and a heterogeneous syndrome with significant variability in its symptoms, causes, severity and response to treatment. It is defined by symptoms such as nausea, vomiting, postprandial fullness and upper abdominal discomfort; objective documentation of delayed gastric emptying of solid food; and exclusion of mechanical obstruction.1
Delayed gastric emptying was first reported in patients with diabetes by Boas2 in 1925, and the term “gastroparesis diabeticorum” was used by Kassander3 in 1958 to describe asymptomatic gastric retention in patients with diabetes. Although diabetes mellitus accounts for more than one-third of all cases of gastroparesis,4 other risk factors include gastrointestinal surgery, medications and neurologic and autoimmune disorders. Moreover, in many patients no underlying cause is found,5 making this condition even more variable.
Regardless of the cause and despite advances in understanding of the pathogenesis (which has unresolved questions), gastroparesis poses a challenge in diagnosis and management for gastroenterologists and nongastroenterologists alike.
This review focuses on the most relevant challenges encountered when approaching patients with this condition, current recommendations for diagnosis and treatment and how nongastroenterologists, such as primary care clinicians, can use these to help manage patients.
The diagnosis of gastroparesis requires three criteria:
Advertisement
There are currently two gold-standard tests to document delayed gastric emptying: gastric emptying scintigraphy and the stable isotope gastric-emptying breath test.
Gastric emptying scintigraphy measures gastric emptying of a solid meal using an egg or protein-based (western-style) or rice-based (Asian-style) meal containing a radioisotope, usually technetium Tc 99m. A gamma camera is used to scan the gastric area (anteroposterior view) at baseline and 30 minutes, one hour, two hours, three hours and four hours after the meal. Normally, more than 90% of the solid meal should be emptied at three hours. Retention of more than 10% at four hours is considered diagnostic for delayed gastric emptying.1 The assessment of severity based on gastric emptying scintigraphy is as follows6:
While this reporting method is the most commonly used, the results can be presented in other ways such as half-time of emptying or rate of emptying (percent per minute), and the test may be conducted under varied protocols. This lack of standardization complicates the clinical utility of the test and poses a challenge for physicians and patients, particularly when interpreting tests from different institutions.6 A unified protocol that can be implemented in all institutions and nuclear medicine facilities would be optimal.
The test has other limitations. Radiation exposure limits its use in pregnant or breastfeeding women; patients with severe symptoms or allergies may not tolerate a solid meal; and special equipment and rooms are needed.4,7 Hyperglycemia can delay gastric emptying and thus confound the test; hence, the test should not be done if the fasting blood glucose level is above 200 mg/dL.4
Advertisement
Medications that affect gastric emptying should be withheld before the test (Table 1).8 This includes marijuana (the time frame is unknown).
Glucagon-like peptide-1 (GLP-1) receptor agonists and gastric inhibitory polypeptide receptor agonists, commonly used for managing diabetes mellitus and obesity, are associated with nausea and vomiting attributed to delayed gastric emptying. However, there are no clear guidelines on how long to withhold these medications before a gastric emptying test. While the American Society of Anesthesiologists advises skipping one weekly dose of GLP-1 receptor agonists before endoscopy, the American Gastroenterological Association at this time does not endorse this recommendation and suggests tailoring the approach for each patient based on the indication for the GLP-1 agonist and clinical symptoms before endoscopy.9 While not officially recommended for gastric emptying scintigraphy, the guidelines above may serve as a reference for clinicians ordering the test. The decision to withhold GLP-1 agonists before gastric emptying scintigraphy seems to be based on institutional guidelines and clinician experience.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9a170299-92c9-4add-82d0-23814b59348e/Gastroparesis-table1)
The stable isotope gastric-emptying breath test involves the patient ingesting a meal containing a carbon 13-labeled substrate such as Arthrospira (Spirulina) platensis (edible blue-green algae) or octanoic acid (a medium-chain fatty acid).10 Then, breath samples are taken to calculate the carbon 13 carbon dioxide excretion rate for approximately four hours, usually at 45, 90, 120, 180 and 240 minutes. At any time point, the carbon 13 carbon dioxide excretion is proportional to the rate of gastric emptying, so that increasing excretion means increasing rates of gastric emptying. Patients with delayed gastric emptying will have carbon 13 carbon dioxide excretion rates lower than references values.10
Advertisement
This test is relatively easy to perform. It can be done in the office or at the bedside and does not require elaborate detection equipment. Because it does not involve radiation exposure, it is safer than scintigraphy, which is especially important in pregnant or breastfeeding women and children.11 On the other hand, it may be inaccurate in patients with malabsorption or liver or lung diseases.1,10 Physical activity influences carbon dioxide production, and hence, measurements of the breath test. Therefore, it is recommended that patients be at rest through the entire test.10
American College of Gastroenterology guidelines1 recommend against using whole-gut motility tests such as the radiopaque marker test as well as the wireless motility capsule to measure gastric emptying. The main reason that the radiopaque marker and the nondigestible wireless motility capsule are not recommended is that they do not empty with the solid food from the stomach and hence may give a false-positive result of delayed gastric emptying.5 There is evidence that the capsule empties during phase III of the migrating motor complex, similar to a nondigestible solid, which occurs after digestion of solid food.12
Electrogastrography may complement the identification of pathophysiologic mechanisms in gastric function, as it reveals distinct patterns and electrical waves associated with specific motility disorders such as gastroparesis, functional dyspepsia and cyclic vomiting. However, the clinical significance of this information remains unclear,1 and as a result, it is not routinely requested. More research will help to clarify its role in clinical practice.
Advertisement
During the assessment, it is important to consider manageable factors that could explain gastroparesis symptoms. This includes reviewing the patient’s medical history, assessing medications that may affect gastric emptying (e.g., opioids, GLP-1 receptor agonists) and obtaining thyroid function tests. In addition, sensory or motor disorders of the upper gastrointestinal tract may have similar symptoms as gastroparesis.
Some functional gastrointestinal disorders can have a clinical presentation similar to that of gastroparesis. Hence, it is important to properly differentiate among them (Table 2).13-15 Functional dyspepsia, rumination syndrome, cyclic vomiting syndrome and others should be considered in the differential diagnosis.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2b058ca8-8ce8-43d2-91cb-b5318213b91c/Gastroparesis-table2)
Functional dyspepsia can be indistinguishable from gastroparesis.13 It is defined by similar symptoms, e.g., early satiety, postprandial fullness, bloating and epigastric discomfort or pain, and approximately 25% to 35% of patients may have delayed gastric emptying.4 Two categories or subtypes are recognized: epigastric pain syndrome and postprandial distress syndrome, with postprandial distress syndrome having more similarities to symptoms of gastroparesis.13
Distinguishing between functional dyspepsia and gastroparesis is important since functional dyspepsia has different treatments and a better prognosis.1
Rumination syndrome presents with effortless, repetitive regurgitation, chewing and reswallowing, or spitting out previously digested food.4,13 It can be diagnosed with combined high-resolution manometry-impedance monitoring, revealing a pattern of low-pressure gastric straining followed by regurgitation.14 Treatment of rumination syndrome is also different, with education and behavioral modification (diaphragmatic and deep-breathing exercises).13,14
Cyclic vomiting syndrome (associated with a personal or family history of migraines) and cannabinoid hyperemesis syndrome (associated with heavy cannabis use) should be considered in the differential diagnosis. Both present with episodic attacks of severe nausea and vomiting, usually associated with dehydration and electrolyte imbalance.4,14
Eating disorders such as anorexia and bulimia nervosa should be considered because a low body mass index is associated with delays in gastric emptying and disturbed gastric functioning. Treatment involves psychotherapy and nutrition enforcement, but not prokinetics.14
Anxiety disorder toward food, also known as avoidant restrictive food intake disorder, can mimic gastroparesis. However, patients with this disorder have immediate nausea and vomiting as soon as they see food (before eating), while those with gastroparesis have delayed symptoms (20 to 30 minutes after eating). This condition is treated with psychotherapy and neuromodulators.13
Narcotic bowel syndrome can be considered in the differential diagnosis, since it is characterized by a progressive and somewhat paradoxical increase in abdominal pain (accompanied by bloating and nausea) despite continued or escalating doses of opioids.14,15
Conditions that present with constipation as the predominant syndrome should also be considered. In this case, upper gastrointestinal symptoms and delayed gastric emptying may be the result of constipation, and the symptoms improve when it resolves.13
A comprehensive strategy for managing gastroparesis includes optimizing nutritional status (balance between nutrients acquired from food and beverages and their use by the body for essential functions), improving gastric emptying, reversing iatrogenic causes and achieving glycemic control in patients with diabetes.1,7,16 It is crucial to avoid medications that exacerbate the gastric emptying delay, such as opioids and GLP-1 receptor agonists. The different strategies for management are summarized in Table 3.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e34ab2c5-267c-48e6-a253-f084f0ad278d/Gastroparesis-table3)
The first-line approach is to educate patients on a small-particle diet.17 This consists of foods with a small particle size or those that can be processed into small particles (eg, soups, smoothies, apple sauce). Foods that are initially not in a small-particle form such as corn, peas and onions should be avoided, but these foods can be included when they are processed to smoothie consistency.17
A registry study found that only one-third of patients with gastroparesis had received nutritional counseling, and just 2% adhered to dietary recommendations for patients with gastroparesis.18 Even though obesity is increasingly prevalent among patients with gastroparesis, 64% of patients in the registry reported consuming calorie-deficient diets, leading to various vitamin and mineral deficiencies.18 Consequently, it is important to include a thorough assessment of caloric intake and provide dedicated nutritional counseling for these patients.
In cases of severe gastroparesis despite medical and nutritional interventions, it may be necessary to consider inserting a jejunal feeding tube to bypass the stomach and deliver the formula directly into the small bowel.16,19 The preferred approach involves placing feeding tubes directly into the jejunum, by either endoscopy or laparoscopy, instead of using percutaneous endoscopic gastrostomy tubes.16 It is crucial to allow for a gradual adaptation period, incrementally increasing the infusion rate over a few days until the desired feeding rate is achieved.16,19 Prolonged use of enteric tubes is typically regarded as safe, but there can be infrequent complications such as clogging, dislodgment, malfunction, tip migration and site infections.19
Patients with severe gastroparesis frequently need hospitalization to address their condition, including intravenous hydration to correct metabolic imbalances, nasoenteric decompression and temporary parenteral nutrition for those experiencing significant weight loss and difficulties with oral intake.7,16 Total parenteral nutrition can be considered for advanced cases of gastroparesis; however, reinstating oral intake is generally recommended when feasible to reduce the risk of complications such as central-line infections.1,16
Pharmacologic therapy of gastroparesis involves prokinetics, antiemetics and neuromodulators. Prokinetics act by stimulating nonsphincteric muscle contractility. They are classified into different pharmacologic classes, including dopamine (D2) receptor antagonists, serotonin (5-hydroxytryptamine 4 [5-HT4]) receptor agonists, cholinesterase inhibitors, motilin-like agents and ghrelin-like agents, although many drugs have multiple mechanisms of action.7,16
Metoclopramide is the only US Food and Drug Administration (FDA)–approved medication for gastroparesis management. It works by blocking D2 receptors and partly activating 5-HT4 receptors, exerting both prokinetic and central antiemetic effects. Initially it enhances gastric emptying of liquids in diabetic gastroparesis, but its symptomatic efficacy is likely secondary to its central antiemetic effect.
Long-term use is limited due to decreasing effectiveness and the risk of central nervous system side effects, including reversible involuntary movements and irreversible tardive dyskinesia. Recent data show a risk of tardive dyskinesia of around 0.1% per 1,000 patient-years.20 Typically, metoclopramide is prescribed at 10 mg three times a day, taken 30 minutes before meals, for a maximum of three months.1,7,16
Metoclopramide is also FDA-approved as a nasal spray for diabetic gastroparesis, offering several advantages such as faster and predictable absorption and better symptom control than the oral preparation. As with the oral preparation, extending treatment with the nasal spray longer than 12 weeks should be avoided.21
Erythromycin is a motilin agonist and enhances gastric emptying when taken orally at a dosage of 250 mg three times a day for one to two weeks. However, its prokinetic effects are restricted by tachyphylaxis after four weeks.16
Domperidone, another D2 antagonist, is as effective as metoclopramide for relief of symptoms, and it does not cross the blood-brain barrier in sufficient quantity to cause the neurologic side effects seen with metoclopramide.7,22 It is typically prescribed at a dosage of 10 mg three times a day. However, it should be used with caution, as it causes relative prolongation of the QTc interval.18 Domperidone is available for prescription through the FDA’s program for expanded access to investigational drugs.1,22
Prucalopride, a 5-HT4 receptor agonist used to treat chronic constipation, recently has been shown to also exert a gastrokinetic effect and to improve symptoms in a relatively small number of patients with idiopathic gastroparesis.23
Several experimental medications are currently in development for the treatment of gastroparesis. These include felcisetrag (a 5-HT4 agonist), tradipitant (a neu-rokinin-1 antagonist), relamorelin (a ghrelin agonist) and trazpiroben (a dopamine D2/D3 receptor antagonist).16
5-HT3 receptor antagonists such as granisetron and ondansetron are known for their effectiveness in managing chemotherapy-induced nausea and vomiting. They reduce nausea without affecting gastric compliance or postprandial accommodation and can be considered for patients with dysmotility disorders primarily characterized by nausea and vomiting.7,16
Neurokinin antagonists like aprepitant and tradipitant have been shown to alleviate nausea.16
Although both marijuana and dronabinol can slow gastric emptying, many patients still turn to THC (tetrahydrocannabinol), found in marijuana, for relief from their nausea.16
Levosulpiride, an antipsychotic agent, promotes gastric emptying through its dual action as an anti-dopaminergic and a 5-HT4 agonist.24
Buspirone, an anxiolytic medication acting as a 5-HT1A agonist, enhances gastric accommodation and alleviates postprandial symptoms independently of its anxiolytic properties.25
Mirtazapine, an antidepressant with central adrenergic and serotonergic effects, has been shown to improve symptoms of nausea, vomiting and loss of appetite.26
Haloperidol, given intravenously, has demonstrated efficacy in reducing abdominal pain and nausea in severely ill patients with gastroparesis in the emergency department.27
Tricyclic antidepressants have generated conflicting data in the context of gastroparesis treatment due to their anticholinergic effects, which could potentially lead to delayed gastric emptying. Notably, nortriptyline demonstrated no discernible difference compared with placebo in patients with idiopathic gastroparesis.28
Gastric electrical stimulation has demonstrated a reduction in the frequency of vomiting, although its mechanism of action remains unclear.1,7
Acupuncture, as a stand-alone treatment or when combined with prokinetic drugs, may offer benefits for symptom management in those with diabetic gastroparesis.
Herbal therapies such as rikkunshito or STW5 are not recommended for the treatment of gastroparesis.1
Brain-gut therapies such as hypnotherapy and cognitive behavioral therapy are widely used in gastrointestinal disorders in which pain and nausea and vomiting are primary symptoms, such as functional dyspepsia, irritable bowel syndrome and rumination syndrome. While it is intuitive to consider their applicability to gastroparesis, evidence supporting their role in gastroparesis treatment is limited. However, given their primary use in patients with anxiety and depression—common comorbidities in gastroparesis—they likely play an important role in gastroparesis management in some patients.29
Both diagnostic (endoscopic functional luminal imaging probe) and therapeutic pyloric interventions (intrapyloric injection of botulinum toxin and pyloromyotomy) are available for gastroparesis. They are indicated in cases of refractory gastroparesis not responding to conservative therapy.
Endoscopic functional luminal imaging probe is an innovative diagnostic method employed to evaluate pyloric function and predict treatment outcomes after peroral pyloromyotomy, also known as gastric peroral endoscopic myotomy (G-POEM).1
Intrapyloric injection of botulinum toxin was initially applied for achalasia and subsequently extended to gastroparesis. However, based on randomized controlled trials, this intervention has not shown symptom improvement and is not recommended for patients with gastroparesis.1,16
Laparoscopic (Heineke-Mikulicz) pyloroplasty involves creating a longitudinal incision across the pylorus, followed by a transverse closure. This surgical approach results in the division of both the longitudinal and circular muscle layers. Laparoscopic pyloroplasty is considered a relatively safe procedure and has been shown to enhance gastric emptying while bringing about short-term improvements in symptoms such as nausea, vomiting, bloating and abdominal pain.1,7,16,30
G-POEM is a novel endoscopic procedure that divides the pylorus from the mucosal surface and presumably cuts predominantly the circular muscle layer while maintaining the longitudinal muscle to avoid perforation.16 G-POEM has been proven effective in treating gastroparesis, leading to improved gastric emptying. It has demonstrated superiority over gastric electrical stimulation for gastroparesis in terms of duration of clinical response (time from the procedure to clinical recurrence, with recurrence defined as symptoms refractory to medical treatment requiring hospitalization along with Gastroparesis Cardinal Symptom Index score ≥ 3 for six months),31 and has shown results equivalent to surgical pyloroplasty in patients with medically refractory gastroparesis.32
REFERENCES
Advertisement
Tips for recognizing a complex condition
Significant improvement in GCSI scores following treatment
Better screening can improve GI outcomes and reduce costs
Promising results could lead to improved screening, better outcomes
Provider vigilance and patient education are key for management
Careful risk stratification is key
Benefits of neoadjuvant immunotherapy reflect emerging standard of care
Multidisciplinary framework ensures safe weight loss, prevents sarcopenia and enhances adherence