Tafamidis is first FDA-approved drug to treat potentially fatal disease
Until 2019, the sole treatment options for people with transthyretin amyloid cardiomyopathy were symptom management and, in some patients, heart transplant. That changed in May, when the FDA approved the small-molecule compound tafamidis for the treatment of this progressive and potentially fatal disease.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
According to results of the phase 3 ATTR-ACT study, tafamidis significantly reduced all-cause mortality at 30 months relative to placebo (30 percent vs. 43 percent) and resulted in fewer cardiovascular-related hospitalizations.
FDA approval of this breakthrough drug may be the first step in turning transthyretin amyloid cardiomyopathy from a life-threatening condition into a manageable chronic disease.
In this video, learn more about why tafamidis is one of Cleveland Clinic’s Top 10 Medical Innovations for 2020.
Video content: This video is available to watch online.
View video online (https://www.youtube.com/embed/oWICeBMTh7c?feature=oembed)
Inaugural Treatment for Transthyretin Amyloid Cardiomyopathy
“Heart failure and atrial fibrillation are two of the most common cardiac diagnoses of the elderly population. Though previously underdiagnosed, it is now clear that a condition called amyloidosis is an important cause of both of these conditions. When amyloidosis affects the heart, it is known as amyloid cardiomyopathy, a progressive and potentially fatal disease that results from destabilized transthyretin, a transport protein. It becomes misfolded and forms amyloid fibrils that deposit into the walls of the heart, stiffening the muscle, eventually leading to heart failure.
Until now, treatment for the condition has been limited to transplant. In May, an approval brought hope to these suffering individuals. Two drug formulations of the compound known as tafamidis have been dubbed the first ever treatment for the two subtypes of the condition, hereditary and wild type. Phase three clinical trial results showed a 30 percent reduced risk of death in patients receiving this breakthrough therapy.
The approval of a drug for these conditions effectively highlights the importance of providing a treatment option where there once was none.”
Advertisement
Advertisement

Advanced software streamlines charting, supports deeper patient connections

How holding simulations in clinical settings can improve workflow and identify latent operational threats

Interactive Zen Quest experience helps promote relaxing behaviors

Cleveland Clinic and IBM leaders share insights, concerns, optimism about impacts
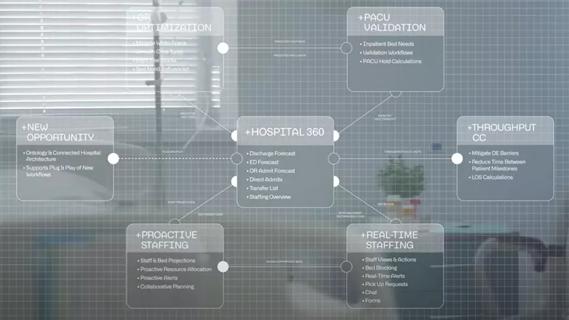
Cleveland Clinic partners with Palantir to create logistical command center

A Q&A with organizational development researcher Gina Thoebes

Cleveland Clinic transformation leader led development of benchmarking tool with NAHQ

Raed Dweik, MD, on change management and the importance of communication