Retrospective study examines accuracy of clinical and laboratory-based biomarker tests
By Mohammad Nasser Kabbany, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
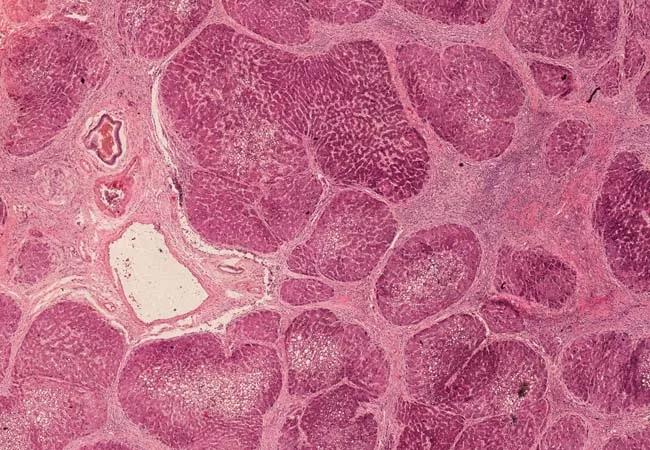
Graft fibrosis after liver transplant is frequently seen in children with a prevalence as high as 64% and up to ten years after transplantation. Graft fibrosis carries a risk of graft dysfunction and subsequent loss, signifying the importance of frequently monitoring liver fibrosis.
Liver biopsy remains the gold standard to stage fibrosis in chronic liver disease in general, particularly in the transplant population. However, it is an invasive procedure that carries a risk of complications. There are ongoing efforts to come up with noninvasive tools to assess liver fibrosis in both adults and children, which include either biomarkers, imaging or both. An ideal screening tool should be accurate, low cost and readily available.
AST/ALT ratio, APRI (AST-platelet-ratio-index) and FIB-4 are markers that are based on readily available clinical and lab data and have been studied extensively in various adult chronic liver diseases with good accuracy in predicting fibrosis stage. However, pediatric studies are lacking especially among liver transplant recipients.
In the largest to date, we conducted a retrospective study in pediatric liver transplant patients younger than 20 years of age at Cleveland Clinic Children’s to assess the accuracy of those scores in predicting the presence of advanced fibrosis (defined as F3-F4) in the allograft compared to biopsy.
We reviewed 232 allograft liver biopsies of which corresponded to 58 transplants performed on 54 patients. The mean age of transplantation was 9.4 ± 7.8 years, and the time between liver transplant and biopsy ranged between a few days and 22 years post-liver transplant with a median of 27 months. All patients were on tacrolimus at the time of biopsy in combination with mycophenolate, steroids or azathioprine.
Advertisement
Advanced fibrosis was noted in 29.3% of patients and in 18.1% of biopsies. The vast majority of biopsies were done as a result of an increase in liver enzymes to rule out rejection. FIB-4 was significantly higher among the advanced fibrosis group compared with mild or no fibrosis. However, FIB-4 had satisfactory accuracy to diagnose advanced fibrosis. We proposed the following FIB-4 cutoff points of 0.2 and 3.03 to rule-in and rule-out advanced fibrosis, respectively. AST/ALT and APRI were not significantly different between patients with and without AF.
The retrospective nature of the study and data that include scores calculated during an acute liver enzyme elevation prevent us from drawing final conclusions about their clinical utility, but they do provide an important basis for future investigations. Prospective study design in a center where allograft liver biopsies are done as part of follow-up protocol will probably better clarify the accuracy of those scores. These tests remain an appealing option because they’re simple, readily available and inexpensive.
There is also growing interest in imaging modalities like velocity-controlled transient elastography and magnetic resonance elastography, which are showing promising results and very good accuracy in fibrosis staging in different liver diseases. However, those imaging modalities are expensive, not widely used, and may require sedation, which can be challenging in children. They also still need to be validated in the pediatric population. Until then, liver biopsy remains the gold standard and the search for improved noninvasive staging tools continues.
Advertisement
About the author: Dr. Kabbany is associate staff in the Department of Pediatric Gastroenterology, Hepatology, and Nutrition at Cleveland Clinic Children’s.
Reference
Habash N, Ezaizi Y, Kak I, et al. Accuracy of noninvasive fibrosis scores in predicting advanced fibrosis in pediatric patients after liver transplantation [published online ahead of print, 2022 Apr 8]. Pediatr Transplant. 2022;e14279. doi:10.1111/petr.14279

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/29bfa6e1-fbae-4595-92dc-8b24f66ea3bd/22-CHP-3431758-Pediatric-2022-Year-in-review-footer-2-7_jpg)
Advertisement
Advertisement

Findings hold lessons for future pandemics

One pediatric urologist’s quest to improve the status quo

Overcoming barriers to implementing clinical trials

Interim results of RUBY study also indicate improved physical function and quality of life

Innovative hardware and AI algorithms aim to detect cardiovascular decline sooner

The benefits of this emerging surgical technology

Integrated care model reduces length of stay, improves outpatient pain management

A closer look at the impact on procedures and patient outcomes