Cleveland Clinic experts weigh in
By: Nitin Aggarwal, MD & Prashanthi N. Thota, MD, FACG
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Conventional treatment for a large symptomatic Zenker diverticulum is to surgically either remove it (diverticulectomy) or obliterate it by repositioning and securing it after cutting into the cricopharyngeus muscle (diverticulopexy with cricopharyngeal myotomy). But high rates of complications with these procedures in elderly patients have led to the development of endoscopic procedures that are safer and have similar or higher success rates.
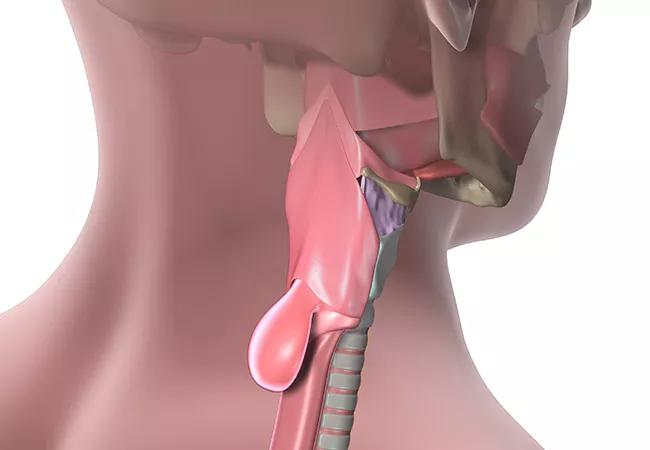
Zenker diverticulum is an outpouching of the esophageal mucosa into the potential space of the Killian triangle, the area between the inferior pharyngeal constrictor and the cricopharyngeus muscle. Increased cricopharyngeal tone coupled with insufficient relaxation results in a pressure gradient that eventually causes esophageal outpouchings. The problem is induced by age-related fibrosis and atrophy, esophageal spasms related to gastroesophageal reflux disease, and idiopathic cricopharyngeal spasms.1
Zenker diverticulum has an estimated incidence of about 2 per 100,000 per year, but this is likely low because many cases are asymptomatic. It occurs mostly in men and people of Northern European descent in their 60s and 70s and rarely before age 40.2
About 80% to 90% of patients present with dysphagia. Other signs and symptoms include regurgitation of undigested foods and medications, halitosis, hoarseness, chronic cough, and aspiration.
Rare complications of Zenker diverticulum include recurrent pulmonary infection, tracheal fistula, ulceration, hemorrhage, squamous cell carcinoma (incidence 0.4% to 1.5%), vocal cord paralysis, and fistula to the prevertebral ligament with cervical osteomyelitis.1
Advertisement
The diagnosis is suspected based on symptoms but should be confirmed with a barium esophagram that shows an outpouching of contrast from the main contrast column.
A watch-and-wait approach should be used for patients with mild symptoms (ie, mild or intermittent dysphagia) and minor functional limitations. Patients should be counseled to eat small amounts of food at a time, to chew thoroughly, and to sip liquids between bites.
Patients with more than mild symptoms who are candidates for intervention should be offered treatment. The goal is to open the septum between the diverticulum and the main esophageal lumen so that food can be propelled from the hypopharynx to the main esophageal lumen without obstruction. Because increased cricopharyngeal tone plays a large role in the development and propagation of a diverticulum, most experts recommend cricopharyngomyotomy in addition to any treatment strategy.
In centers that do not offer flexible endoscopy, and for patients with a large diverticulum, open surgery is the sole option. It is done under general anesthesia with a left cervical approach. The length of the cricopharyngeal myotomy can vary from 2 to 6 cm.3,4 This is followed by one of three options:
Success rates for open surgery vary from study to study but are usually around 90%.4 Complications are reported in 10% to 30% of cases and include mediastinitis, severe recurrent laryngeal nerve injury, and a 1% to 2% chance of death. These rates seem high, but the older age of most of these patients puts them at high risk.5
Advertisement
All endoscopic procedures involve incision of the wall separating the diverticulum from the esophageal lumen to relieve the obstruction.
Rigid endoscopy is used in centers that do not offer flexible endoscopy for patients who are not candidates for surgery. It is done under general anesthesia. With the patient’s neck completely hyperextended, a rigid endoscope is passed into the oral cavity, and diverticulotomy and cricopharyngeal myotomy are performed.
This technique has been extensively evaluated and, although effective, carries up to an 8% risk of complications, including perforation. It is not recommended for patients with a small diverticulum, a high body mass index, or difficulty with neck extension.6
Flexible endoscopy is the first-line therapy for most patients in many centers. With the flexible endoscope, the diverticulotomy and the cricopharyngeal myotomy can be performed in an outpatient endoscopy suite, and general anesthesia is not required.3
Initial studies of outcomes using these approaches have been promising,3,7–9 with substantial reduction in dysphagia, regurgitation, and chronic cough. Unfortunately, this technique is associated with a recurrence rate as high as 25%. However, repeat endoscopic therapy in patients with recurrence results in success rates similar to those for first-time treatment.
Complication rates are low. In a series of 150 patients, four adverse events occurred—three cases of fever and one of pneumonia. In addition, one patient had subcutaneous emphysema that resolved spontaneously.7
Advertisement
A potentially major advance in the endoscopic treatment of Zenker diverticulum is the development of a diverticuloscope, a soft tube with a V-shaped end. This tube is inserted through the mouth with one leg of the “V” protecting the anterior esophageal wall and the other leg protecting the posterior diverticular lumen. The endoscope is passed through this tube to perform the diverticulotomy. In one report,10 diverticuloscope-guided diverticulotomy led to fewer complications, shorter procedural time, and higher success rates compared with the standard technique.10 This device is not yet available in the United States.
No head-to-head comparison of outcomes with various flexible endoscopic techniques for treating Zenker diverticulum has been published, nor are there data yet on the success of surgery if endoscopic therapy fails.
We recommend flexible endoscopy for the initial treatment of Zenker diverticulum in most patients, but its availability is limited in the United States, as many practitioners do not have adequate experience with the technique.
Advertisement
This article originally appeared in the Cleveland Clinic Journal of Medicine. 2016 September;83(9):645-647
Nitin Aggarwal, MD, Former Fellow, Department of Gastroenterology and Hepatology, Digestive Disease & Surgery Institute, Cleveland Clinic
Prashanthi N. Thota, MD, FACG, Director, Esophageal Center and Director, Center of Excellence for Barrett’s Esophagus, Department of Gastroenterology and Hepatology, Digestive Disease & Surgery Institute, Cleveland Clinic
Advertisement

Multidisciplinary framework ensures safe weight loss, prevents sarcopenia and enhances adherence

Study reveals key differences between antibiotics, but treatment decisions should still consider patient factors

Key points highlight the critical role of surveillance, as well as opportunities for further advancement in genetic counseling

Potentially cost-effective addition to standard GERD management in post-transplant patients

Findings could help clinicians make more informed decisions about medication recommendations

Insights from Dr. de Buck on his background, colorectal surgery and the future of IBD care

Retrospective analysis looks at data from more than 5000 patients across 40 years

Surgical intervention linked to increased lifespan and reduced complications