Snapshots of four interventional therapies in clinical testing
For patients with heart failure, the treatment landscape between guideline-directed medical therapy (GDMT) and left ventricular assist device (LVAD) placement or heart transplant can be broad and challenging. Persistent symptoms despite GDMT substantially undermine quality of life and lead to frequent hospital admissions.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
To make this landscape more navigable, clinician-researchers are increasingly exploring interventional approaches for the many patients whose heart failure is inadequately controlled by GDMT. While all of these interventional approaches involve devices that are still investigational in the United States, many are showing considerable potential for clinical use. Cleveland Clinic is leading or otherwise involved in clinical trials of at least four of these devices and approaches, which are illustrated in Figure 1 and profiled in the story below.
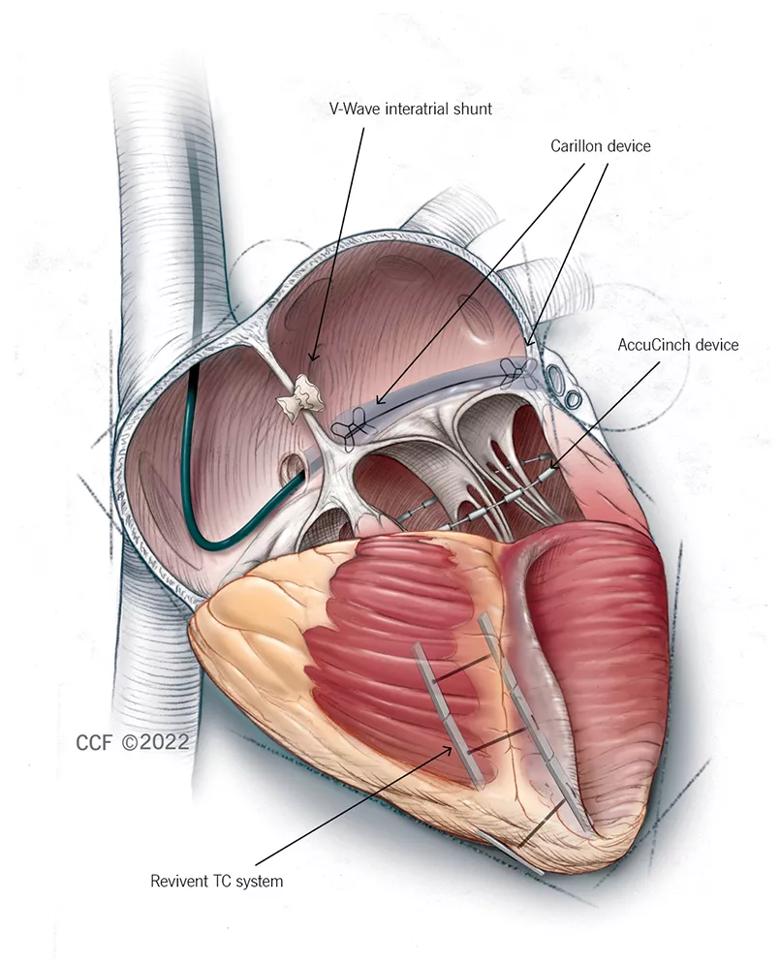
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f017b7d0-6995-47f7-ade8-6907dc6462af/22-HVI-2770308-interventional-HF-inset-1_jpg)
Figure 1. Illustration of the four featured interventional approaches. The V-Wave shunt diverts blood across the interatrial septum from the left to the right atrium to reduce filling pressure. The Carillon device is cinched and anchored in the coronary sinus to reshape the mitral valve annulus and reduce mitral annular dilation. The AccuCinch device reduces left ventricular wall stress and dimensions to improve myocardial contractility and overall function. The Revivent TC system is used to exclude the scar in ischemic cardiomyopathy with left ventricular scarring, thereby reducing left ventricular volume and wall stress. ©2022 The Cleveland Clinic Foundation. All rights reserved.
One interventional approach to heart failure involves placement of the Carillon Mitral Contour System, which has CE mark approval in Europe for treatment of functional mitral regurgitation (MR). The Carillon device is placed in the right heart via transcatheter access through the jugular vein. After measurement of the coronary sinus to guide device size selection, the device is cinched and anchored in the coronary sinus to reshape the mitral valve annulus and reduce mitral annular dilation — and thereby theoretically improve heart function.
Advertisement
Although the Carillon device is not currently approved for functional MR in the U.S., its investigation for use in heart failure can be partly traced back to Cleveland Clinic’s role as a core lab in some initial trials of Carillon for severe functional MR. “When our core lab experts read the echocardiograms, we saw that some of the patients did not have very much MR to begin with, and their ventricles were dilated,” explains Samir Kapadia, MD, Chair of Cardiovascular Medicine at Cleveland Clinic. Post hoc analysis revealed that patients who received the device showed improvements in New York Heart Association (NYHA) class, Kansas City Cardiomyopathy Questionnaire (KCCQ) score, functional status and hemodynamics.
These secondary findings prompted Dr. Kapadia, an interventional cardiologist, and his Cleveland Clinic colleague Randall Starling, MD, MPH, of the Section of Heart Failure and Cardiac Transplant Medicine, to propose a multicenter study of the device in patients with any degree of functional MR — including mild and moderate — so long as they also had heart failure. “We felt it would be a reasonable strategy to try to treat the heart failure, not specifically the valve disease,” Dr. Kapadia says. The resulting study is the EMPOWER trial (NCT03142152), for which Drs. Kapadia and Starling are serving as national principal investigators (PIs).
The international trial, which launched last year, is expected to enroll 300 patients who have heart failure with at least mild functional MR — a population estimated to represent about 30% to 50% of all heart failure patients in the United States. “This is an extremely large patient population that is not currently being addressed by studies of other novel therapies,” Dr. Kapadia notes.
Advertisement
Patients will be randomized 1:1 in a double-blind manner to receive the Carillon device or a sham control device. The study has primary safety and efficacy endpoints at 12 months and will follow patients out to five years.
“For a patient to be eligible for EMPOWER, they must be evaluated by a heart failure cardiologist who reviews the adequacy of the guideline-directed medical therapy,” says Dr. Starling. “Our hope is that combining medications with the Carillon device will reshape the left ventricle so that it becomes smaller and there is less mitral regurgitation.”
The same general concept underlying the Carillon device — cinching the upper part of the heart to improve heart function — applies to another interventional heart failure treatment now in clinical testing, the AccuCinch® Ventricular Restoration System.
“AccuCinch is the first fully transcatheter therapy designed to restore, support and strengthen the dilated left ventricle in the setting of heart failure with reduced ejection fraction (HFrEF),” says Rishi Puri, MD, PhD, an interventional cardiologist who serves as Cleveland Clinic’s site PI for a randomized controlled trial of AccuCinch known as CORCINCH-HF (NCT04331769).
The device is implanted via transfemoral access with a 20-Fr femoral arterial sheath. Placement involves retrograde aortic access with catheters specially designed to navigate around the chords at the base of the left ventricle. That provides a roadmap for positioning a guidewire to place and stitch in multiple anchors along the ventricle perimeter and then cinch, significantly reducing the diameter at the base of the ventricle between the septum and lateral wall.
Advertisement
“The aim is to reduce left ventricular wall stress and dimensions, initiating a biologic process of reverse remodeling to improve myocardial contractility and overall function,” says Dr. Puri. “This should improve the patient’s quality of life and survival, increase exercise capacity and reduce heart failure hospitalizations.”
He notes that implantation can take 90 minutes to three hours and involves a workflow similar to that of transcatheter mitral annuloplasty procedures.
The open-label CORCINCH-HF study is randomizing 400 patients at up to 80 centers to treatment with AccuCinch plus GDMT or GDMT alone. Patients must have symptomatic HFrEF (20% to 40%) despite GDMT, as well as a left ventricular end diastolic diameter ≥ 55 mm. The study has safety, clinical efficacy and quality-of-life endpoints at six and 12 months and will follow patients for at least two years.
Like the Carillon device, AccuCinch was initially designed to treat functional MR, and early feasibility studies in Europe suggested its utility for HFrEF as well. Dr. Puri says that data from those studies showed 20-point improvements in quality of life as measured by KCCQ score. “Data with procedures such as MitraClip™ and transcatheter aortic valve replacement suggest that improvements of 10 or more points in KCCQ score tend to correlate with improved mortality and other outcomes,” he notes. “That gives encouragement about the potential benefits of the AccuCinch procedure.”
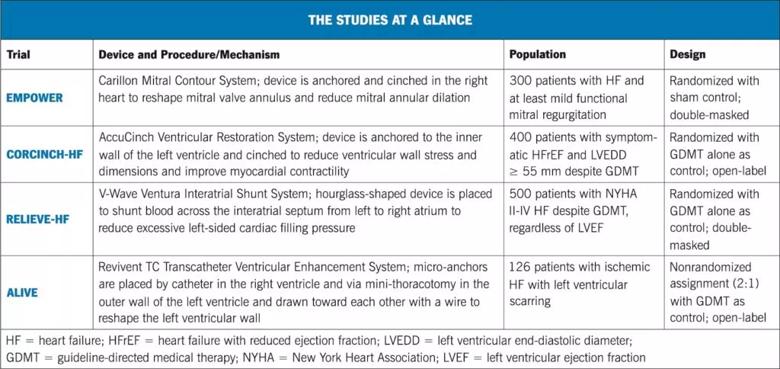
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7bb86f88-d000-464a-9c01-160c84a306e5/22-HVI-2770308_Inset-2-1024x484_jpg)
Decompression of the heart is again the concept behind another interventional strategy — the V-Wave Ventura® Interatrial Shunt System.
Advertisement
This approach involves implantation of an hourglass-shaped device designed to shunt blood across the interatrial septum from the left atrium to the right atrium. The aim is to reduce excessive left-sided cardiac filling pressure in the setting of advanced heart failure, thereby improving symptoms related to pulmonary congestion.
The shunt is placed using a right-sided femoral catheterization procedure under fluoroscopic and echocardiographic guidance. The shunt’s hourglass design holds it in place and makes blood transfer more efficient, enabling a smaller shunt size. “This is important because a previous interatrial shunt, which was larger, did not perform well in clinical trials, and this was believed to be due in part to its size,” notes Dr. Kapadia.
The new shunt system is being evaluated in the multicenter RELIEVE-HF study (NCT03499236), in which 500 patients are being randomized 1:1 to either shunt placement plus GDMT or GDMT alone. To enable masking, all patients — including controls — undergo diagnostic right heart catheterization and invasive echocardiography, which is followed by shunt placement only in those randomized to the intervention arm. Eligible patients include those with NYHA class II, class III or ambulatory class IV disease despite maximally tolerated GDMT, regardless of left ventricular ejection fraction.
Primary endpoints are major device-related adverse events at 30 days and a composite of death, heart transplant/LVAD implantation, heart failure hospitalization, outpatient treatment of worsening heart failure and change in KCCQ score at one and two years. Shunt recipients will be followed for five years.
In addition to participating in RELIEVE-HF, Dr. Kapadia and his interventional cardiology colleague Grant Reed, MD, MSc, are conducting a related investigation for which they obtained an investigational device exemption from the FDA. The open-label study (NCT04729933) is assessing the safety and feasibility of implanting the interatrial shunt immediately following percutaneous mitral valve repair with the MitraClip device.
Patients are similar to those in RELIEVE-HF except that they also have at least moderate to severe functional MR. “Even with MitraClip treatment and maximum guideline-directed medical therapy, these patients are at high risk for recurrent heart failure events and admissions, so they represent an unmet need for further therapies,” Dr. Reed explains. “Since we are already performing MitraClip through the interatrial septum, the same transseptal puncture can be used to place the V-Wave device. This makes the interatrial shunt permanent and eliminates the need for an additional procedure.”
The 10-patient study is being conducted exclusively at Cleveland Clinic. If results are promising, further study will be pursued. Meanwhile, primary completion of RELIEVE-HF is expected at the end of 2022.
A fourth interventional approach is being investigated for a more narrowly defined subpopulation of heart failure patients. The Revivent TC™ Transcatheter Ventricular Enhancement System has been developed for patients referred for surgical treatment of ischemic cardiomyopathy with left ventricular scarring that is contiguous and includes both anterior and septal components.
Such patients may be considered for left ventricular reconstructive surgery with the Dor procedure, but this requires an open heart approach and cardiopulmonary bypass. “We are hopeful that Revivent TC will be shown to offer a better alternative for these patients whom we are hesitant to recommend for highly invasive surgery,” says Edward Soltesz, MD, MPH, Surgical Director of Cleveland Clinic’s Kaufman Center for Heart Failure Treatment and Recovery. “It’s anticipated that this less-invasive approach will slow cardiomyopathy progression and improve quality of life.”
The Revivent TC procedure involves hybrid placement of two sets of micro-anchors in the scarred heart. Internal micro-anchors are placed into the interventricular septum of the right ventricle by an interventional cardiologist with transcatheter access via the internal jugular vein. External micro-anchors are placed in the outer wall of the left ventricle below the scar tissue by a cardiac surgeon via a 4-cm mini-thoracotomy. When the micro-anchor pairs are drawn toward each other with a wire, the newly shaped left ventricular wall consists of functioning tissue and takes on a more normal shape and size.
In the wake of promising European data, the Revivent TC procedure is being studied in a U.S. trial known as ALIVE (NCT02931240), which is enrolling 126 patients assigned 2:1 to Revivent TC versus GDMT. Enrollment criteria are:
Primary safety endpoints include all-cause death, placement of a mechanical support device and bleeding or tamponade at 30 days and one year. The primary effectiveness endpoint is a composite of freedom from readmission and improvements in quality-of-life score, six-minute walk distance and NYHA class. Patients will be followed through five years.
“The Revivent TC procedure offers a minimally invasive option to select patients with symptomatic ischemic heart failure without obviating advanced heart failure treatment options in the future,” says the ALIVE trial’s national co-principal investigator, Jerry Estep, MD, Chair of Cardiovascular Medicine at Cleveland Clinic Florida. “If the trial results are positive, it will provide an appealing solution to the scar tissue that is the root cause of left ventricular dysfunction and disease progression in these patients.”
Dr. Kapadia says several of these emerging interventional therapies for HF stand out in various ways:
From his cardiac surgery perspective, Dr. Soltesz says that all four interventional approaches are “quite promising” and share a common advantage. “Since all are performed minimally invasively,” he notes, “they do not obviate any advanced therapy options, such as heart transplant or LVAD implant, when these eventually become necessary.”
Dr. Kapadia adds that whereas these approaches focus on the heart itself, additional research is pursuing interventional therapies designed to address effects of heart failure on other body systems, such as ways to diurese the kidney to promote better kidney function. “Investigators are looking at sympathetic stimulation, nerve blockage, stimulating the ganglia, carotid stimulation,” he says. “The focus of efforts is expanding to manifestations of heart failure throughout the rest of the body.”
Whatever the targeted mechanism, Dr. Kapadia notes, new interventions for heart failure must get the risk-benefit calculation right. “The risk of new heart failure treatments must be low, because this is a patient population that can go downhill very fast,” he concludes. “Safety is paramount in this realm.”
Advertisement
Modified-Bentall single-patch Konno enlargement (BeSPoKE) optimizes hemodynamics, facilitates future TAVR
Cleveland Clinic’s new dedicated program offers nuanced care for a newly recognized cardiovascular risk factor
Scenarios where experience-based management nuance can matter most
Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics
How Cleveland Clinic is using and testing TMVR systems and approaches
NIH-funded comparative trial will complete enrollment soon
How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR
Optimal management requires an experienced center