Minimally invasive percutaneous/surgical procedure reshapes the heart
The multicenter ALIVE (American Less Invasive Ventricular Enhancement) trial is underway to evaluate the safety and efficacy of the Revivent TC™ Transcatheter Ventricular Enhancement System for heart failure patients with left ventricular scarring. The system requires a cardiac surgeon and an interventional cardiologist working together simultaneously to reshape the heart to a more normal size, with the goal of improving pumping efficiency.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Interventional heart failure therapies are the next frontier of invasive cardiology,” says Cleveland Clinic interventional cardiologist Rishi Puri, MD, PhD, the trial’s local principal investigator. “The Revivent system has the capacity to alter the treatment paradigm for a population of patients with heart failure who have very limited treatment options.”
According to Edward Soltesz, MD, MPH, Surgical Director of Cleveland Clinic’s Kaufman Center for Heart Failure and Recovery, many patients with heart failure have poorly controlled symptoms despite medical therapy but do not yet have disease severe enough to warrant a heart transplant or left ventricular assist device. For such patients, left ventricular reconstructive surgery using the Dor procedure may be indicated, requiring open-heart surgery and cardiopulmonary bypass.
“We are optimistic that the ALIVE trial will lead to a better alternative for these patients whom we are hesitant to recommend for highly invasive surgery,” says Dr. Soltesz. “It’s anticipated that the system used in this trial will slow cardiomyopathy progression and improve quality of life.”
Less Invasive Ventricular Enhancement (LIVE™ Therapy) using the Revivent system was approved for use in Europe in 2016. Evidence indicates that it increases ejection fraction, reduces ventricular volume and improves New York Heart Association (NYHA) functional class and exercise capacity. Long-term outcomes data are not yet available.
The Revivent procedure is performed off pump and without a ventriculotomy. It involves placement of the following:
Advertisement
Usually three pairs of internal/external micro-anchors are needed. When the micro-anchor pairs are drawn toward each other with a wire, the newly shaped left ventricular wall consists of functioning tissue and is of a more normal size and shape. Patients typically remain in the hospital three to four days after the procedure.
The trial is anticipated to enroll 126 patients in up to 30 sites in the U.S., with 84 patients in the intervention arm and 42 controls who do not undergo the procedure.
Enrollment criteria include the following:
Exclusion criteria include having a cardiac resynchronization therapy device placed within 60 days, peak systolic pulmonary artery pressure > 60 mm Hg, myocardial infarction within 90 days or chronic renal failure (serum creatinine > 2.5 mg/dL or glomerular filtration rate < 30 mL/min). Patients with prior pericardiotomy, left thoracotomy or open-heart surgery do not qualify for the intervention but may enroll as control subjects.
Patients are followed for 12 months. Efficacy is being evaluated in terms of:
Safety is being assessed in terms of:
Advertisement
Preliminary results are anticipated at the end of 2021.
The investigators expect this trial will be definitive for this method of scar elimination.
They note that the high degree of procedural collaboration between interventional cardiologist and cardiac surgeon required by the Revivent approach is unusual in cardiac therapy. They add that Cleveland Clinic’s Kaufman Center for Heart Failure Treatment and Recovery is well suited for such collaboration, given that it already serves as an umbrella structure under which multidisciplinary subspecialists routinely work together to optimize patient outcomes.
Cleveland Clinic completed the procedure in one ALIVE trial enrollee in late 2020 (Figure) and anticipates enrolling several additional patients this year.
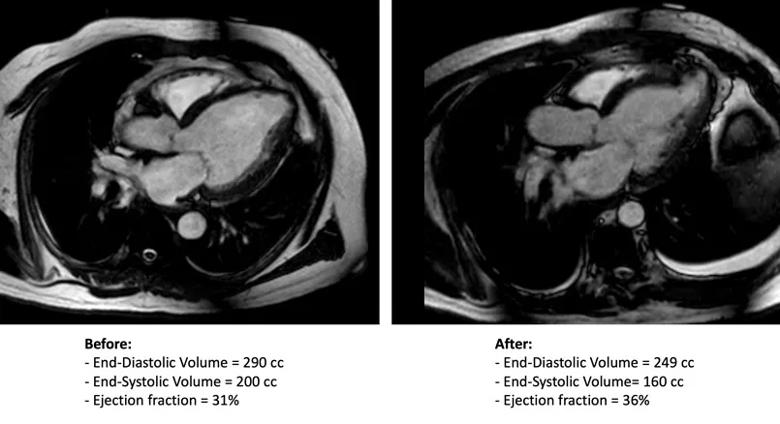
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/68fb9f31-d778-4616-969f-dc555af26481/20-HVI-2034259-CQD-ALIVE-study-profile-inset_jpg)
Figure. Axial MRIs taken before (left) and after (right) the Revivent procedure.
“The Revivent procedure offers a minimally invasive treatment option to patients with symptomatic heart failure without obviating advanced heart failure treatment options in the future,” adds the ALIVE trial’s national co-principal investigator Jerry Estep, MD, Medical Director of Cleveland Clinic’s Kaufman Center for Heart Failure and Recovery and Section Head of Heart Failure and Transplantation. “If the trial results are positive, this will be a game changer and will add to our heart failure therapeutic armamentarium.”
Advertisement
Advertisement

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients