A closer look at AD drug therapies

By Luke D. Kim, MD, FACP, CMD, and Ronan M. Factora, MD, FACP, AGSF
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Alzheimer disease is the most common form of dementia. In 2016, an estimated 5.2 million Americans age 65 and older had Alzheimer disease. The prevalence is projected to increase to 13.8 million by 2050, including 7 million people age 85 and older.1
Although no cure for dementia exists, several cognition-enhancing drugs have been approved by the US Food and Drug Administration (FDA) to treat the symptoms of Alzheimer dementia. The purpose of these drugs is to stabilize cognitive and functional status, with a secondary benefit of potentially reducing behavioral problems associated with dementia.
CURRENTLY APPROVED DRUGS
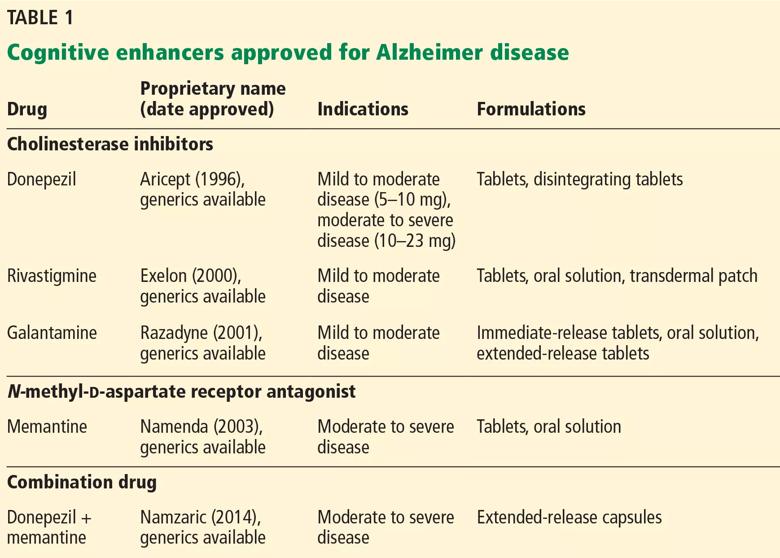
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c30fdda0-c850-4040-b3e1-0eb96cec364e/GER-AD-REALtable1_jpg)
Two classes of drugs are approved to treat Alzheimer disease: cholinesterase inhibitors and an N-methyl-d-aspartate (NMDA) receptor antagonist (Table 1).
The cholinesterase inhibitors act by reversibly binding and inactivating acetylcholinesterase, consequently increasing the time the neurotransmitter acetylcholine remains in the synaptic cleft. The 3 FDA-approved cholinesterase inhibitors are donepezil, galantamine, and rivastigmine. Tacrine, the first approved cholinesterase inhibitor, was removed from the US market after reports of severe hepatic toxicity.2
The clinical efficacy of cholinesterase inhibitors in improving cognitive function has been shown in several randomized controlled trials.3–10 However, benefits were generally modest, and some trials used questionable methodology, leading experts to challenge the overall efficacy of these agents.
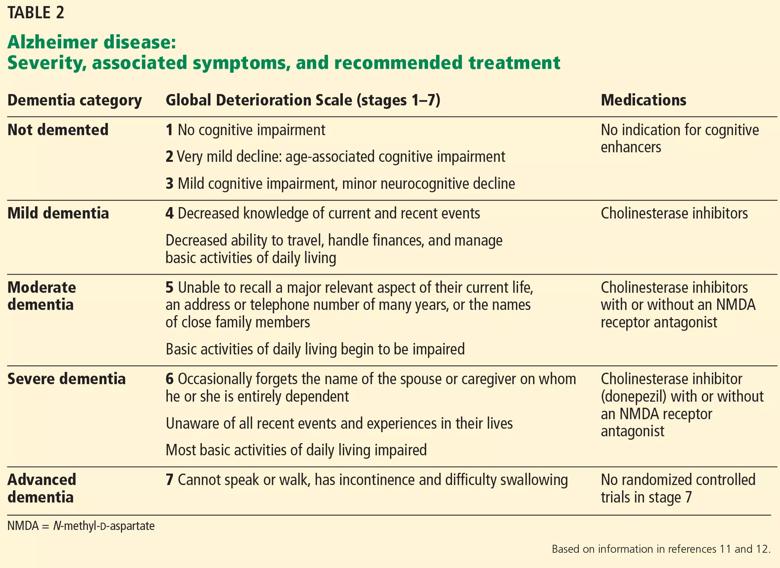
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/45f3d968-73a4-4e2f-bfbc-0ae676faca1a/GER-AD-table1_jpg)
All 3 drugs are approved for mild to moderate Alzheimer disease (stages 4–6 on the Global Deterioration Scale; Table 2)11,12; only donepezil is approved for severe Alzheimer disease. Rivastigmine has an added indication for treating mild to moderate dementia associated with Parkinson disease. Cholinesterase inhibitors are often used off-label to treat other forms of dementia such as vascular dementia, mixed dementia, and dementia with Lewy bodies.13
Advertisement
Memantine, currently the only FDA-approved NMDA receptor antagonist, acts by reducing neuronal calcium ion influx and its associated excitation and toxicity. Memantine is approved for moderate to severe Alzheimer disease.
Often, these 2 classes of medications are prescribed in combination. In a randomized controlled trial that added memantine to stable doses of donepezil, patients had significantly better clinical response on combination therapy than on cholinesterase inhibitor monotherapy.14
In December 2014, the FDA approved a capsule formulation combining donepezil and memantine to treat symptoms of Alzheimer dementia. However, no novel pharmacologic treatment for Alzheimer disease has been approved since 2003. Furthermore, recently Pfizer announced a plan to eliminate 300 research positions aimed at finding new drugs to treat Alzheimer disease and Parkinson disease.15
CONSIDERATIONS WHEN STARTING COGNITIVE ENHANCERS
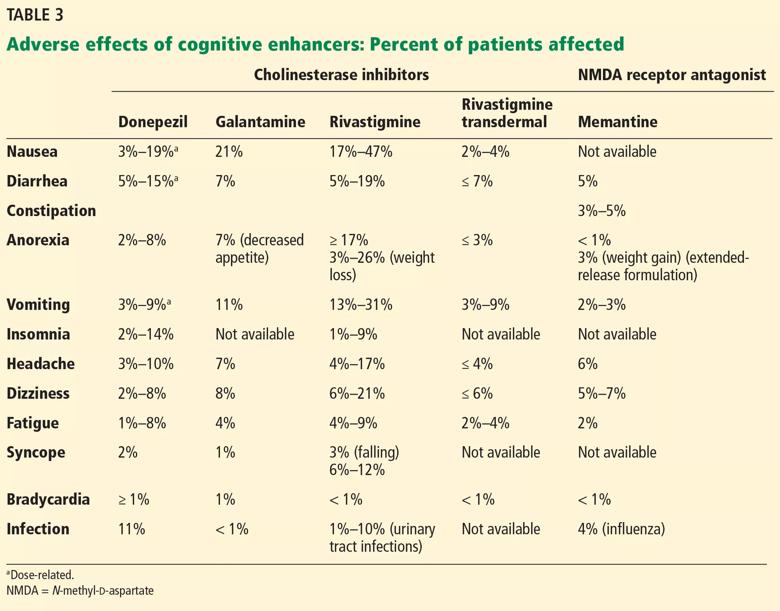
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d797b1a3-54e4-4426-b387-3f2b9646ca09/GER-AD-table3_jpg)
Adverse effects of cholinesterase inhibitors are generally mild and well tolerated and subside within 1 to 2 weeks. Gastrointestinal effects are common, primarily diarrhea, nausea, and vomiting. They are transient but can occur in about 20% of patients (Table 3).
Other potential adverse effects include bradycardia, syncope, rhabdomyolysis, neuroleptic malignant syndrome, and esophageal rupture. Often, the side-effect profile helps determine which patients are appropriate candidates for these medications.
As expected, higher doses of donepezil (23 mg vs 5–10 mg) are associated with higher rates of nausea, diarrhea, and vomiting.
Advertisement
Dosing. The cholinesterase inhibitors should be slowly titrated to minimize side effects. Starting at the lowest dose and maintaining it for 4 weeks allows sufficient time for transient side effects to abate. Some patients may require a longer titration period.
As the dose is escalated, the probability of side effects may increase. If they do not subside, dose reduction with maintenance at the next lower dose is appropriate.
Gastrointestinal effects. Given the adverse gastrointestinal effects associated with this class of medications, patients experiencing significant anorexia and weight loss should generally avoid cholinesterase inhibitors. However, the rivastigmine patch, a transdermal formulation, is an alternative for patients who experience gastrointestinal side effects.
Bradycardia risk. Patients with significant bradycardia or who are taking medications that lower the heart rate may experience a worsening of their bradycardia or associated symptoms if they take a cholinesterase inhibitor. Syncope from bradycardia is a significant concern, especially in patients already at risk of falls or fracture due to osteoporosis.
The side-effect profile of memantine is generally more favorable than that of cholinesterase inhibitors. In clinical trials, it has been better tolerated with fewer adverse effects than placebo, with the exception of an increased incidence of dizziness, confusion, and delusions.16,17
Caution is required when treating patients with renal impairment. In patients with a creatinine clearance of 5 to 29 mL/min, the recommended maximum total daily dose is 10 mg (twice-daily formulation) or 14 mg (once-daily formulation).
Advertisement
These medications have been used off-label to treat behavioral problems associated with dementia. A systematic review and meta-analysis showed cholinesterase inhibitor therapy had a statistically significant effect in reducing the severity of behavioral problems.18 Unfortunately, the number of dropouts increased in the active-treatment groups.
Patients with behavioral problems associated with dementia with Lewy bodies may experience a greater response to cholinesterase inhibitors than those with Alzheimer disease.19Published post hoc analyses suggest that patients with moderate to severe Alzheimer disease receiving memantine therapy have less severe agitation, aggression, irritability, and other behavioral disturbances compared with those on placebo.20,21 However, systematic reviews have not found that memantine has a clinically significant effect on neuropsychiatric symptoms of dementia.18,22,23
In early randomized controlled trials, adding memantine to a cholinesterase inhibitor provided additional cognitive benefit in patients with Alzheimer disease.15,24 However, a more recent randomized controlled trial did not show significant benefits for combined memantine and donepezil vs donepezil alone in moderate to severe dementia.25
In patients who had mild to moderate Alzheimer disease at 14 Veterans Affairs medical centers who were already on cholinesterase inhibitor treatment, adding memantine did not show benefit. However, the group receiving alpha-tocopherol (vitamin E) showed slower functional decline than those on placebo.26 Cognition and function are not expected to improve with memantine.
Advertisement
CONSIDERATIONS WHEN STOPPING COGNITIVE ENHANCERS
The cholinesterase inhibitors are usually prescribed early in the course of dementia, and some patients take these drugs for years, although no studies have investigated benefit or risk beyond 1 year. It is generally recommended that cholinesterase inhibitor therapy be assessed periodically, eg, every 3 to 6 months, for perceived cognitive benefits and adverse gastrointestinal effects.
These medications should be stopped if the desired effects—stabilizing cognitive and functional status—are not perceived within a reasonable time, such as 12 weeks. In some cases, stopping cholinesterase inhibitor therapy may cause negative effects on cognition and neuropsychiatric symptoms.27
Deciding whether benefit has occurred during a trial of cholinesterase inhibitors often requires input and observations from the family and caregivers. Soliciting this information is key for practitioners to determine the correct treatment approach for each patient.
Although some patients with moderately severe disease experience clinical benefits from cholinesterase inhibitor therapy, it is reasonable to consider discontinuing therapy when a patient has progressed to advanced dementia with loss of functional independence, thus making the use of the therapy—ie, to preserve functional status—less relevant. Results from a randomized discontinuation trial of cholinesterase inhibitors in institutionalized patients with moderate to severe dementia suggest that discontinuation is safe and well tolerated in most of these patients.28
Abruptly stopping high-dose cholinesterase inhibitors is not recommended. Most clinical trials tapered these medications over 2 to 4 weeks. Patients taking the maximum dose of a cholinesterase inhibitor should have the dose reduced to the next lowest dose for 2 weeks before the dose is reduced further or stopped completely.
CONSIDERATIONS FOR OTHER DEMENTIA THERAPY
Behavioral and psychiatric problems often accompany dementia; however, no drugs are approved to treat these symptoms in patients with Alzheimer disease.
Nonpharmacologic interventions are recommended as the initial treatment.29 Some practitioners prescribe psychotropic drugs off-label for Alzheimer disease, but most clinical trials have not found these therapies to be very effective for psychiatric symptoms associated with Alzheimer disease.30,31
Recently, a randomized controlled trial of dextromethorphan-quinidine showed mild reduction in agitation in patients with Alzheimer disease, but there were significant increases in falls, dizziness, and diarrhea.32
Patients prescribed medications for behavioral and psychological symptoms of dementia should be assessed every 3 to 6 months to determine if the medications have been effective in reducing the symptoms they were meant to reduce. If there has been no clear reduction in the target behaviors, a trial off the drug should be initiated, with careful monitoring to see if the target behavior changes. Dementia-related behaviors may worsen off the medication, but a lower dose may be found to be as effective as a higher dose. As dementia advances, behaviors initially encountered during one stage may diminish or abate.
In a long-term care setting, a gradual dose-reduction trial of psychotropic medications should be conducted every year to determine if the medications are still necessary.33 This should be considered during routine management and follow-up of patients with dementia-associated behavioral problems.
REASONABLE TO TRY
Cognitive enhancers have been around for more than 10 years and are reasonable to try in patients with Alzheimer disease. All the available drugs are FDA-approved for reducing dementia symptoms associated with mild to moderate Alzheimer disease; donepezil and memantine are also approved for severe Alzheimer disease, either in combination or as monotherapy.
When selecting a cognitive enhancer, practitioners need to consider the potential for adverse effects. And if a cholinesterase inhibitor is prescribed, it is important to periodically assess for perceived cognitive benefits and adverse gastrointestinal effects. The NMDA receptor antagonist has a more favorable side effect profile. Combining the drugs is also an option.
Similarly, patients prescribed psychotropic medications for behavioral problems related to dementia should be reassessed to determine if the dose could be reduced or eliminated, particularly if targeted behaviors have not responded to the treatment or the dementia has advanced.
For patients on cognitive enhancers, discontinuation should be considered when the dementia advances to the point where the patient is totally dependent for all basic activities of daily living, and the initial intended purpose of these medications—preservation of cognitive and functional status—is no longer achievable.
This article first appeared in Cleveland Clinic Journal of Medicine. 2018 March;85(3):209-214
Advertisement

Researchers explore the mental and physical benefits of social prescribing

Multidisciplinary approach helps address clinical and psychosocial challenges in geriatric care

Effective screening, advanced treatments can help preserve quality of life

Study suggests inconsistencies in the emergency department evaluation of geriatric patients

Auditory hallucinations lead to unusual diagnosis

How providers can help prevent and address this under-reported form of abuse

How providers can help older adults protect their assets and personal agency

Recognizing the subtle but destructive signs of psychological abuse in geriatric patients