Third annual report shows shift toward prevention studies, disease-modifying therapies
Cleveland Clinic’s third annual analysis of Alzheimer’s disease (AD) drug development shows that progress continues to be disappointingly slow. However, researchers are finding a variety of new drug targets and testing agents earlier in the disease course, the report finds. Importantly, it also reveals that most new drugs are aimed at disease modification rather than symptomatic improvement.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The report, titled “Alzheimer’s Disease Drug Development: Pipeline 2018,” is based on analysis of the federal website clinicaltrials.gov and appears as a feature article in Alzheimer’s & Dementia: Translational Research & Clinical Interventions.
While the AD drug development pipeline is slightly larger in 2018 than in 2017, the authors note that there’s urgent need for a disease-modifying therapy that prevents or delays onset of the disease or slows its progression. Even a one-year delay in onset by 2020 would result in 9.2 million fewer cases of AD by 2050, they observe.
Of the 112 agents in the pipeline, 63 percent are disease-modifying therapies. The analysis revealed a shift toward testing of candidate drugs in people earlier in the disease course, such as individuals with mild cognitive impairment or even preclinical populations — i.e., cognitively normal individuals at high risk for developing AD. The visual abstract below breaks down the pipeline’s 26 late-stage (phase 3) trials by targeted mechanism.
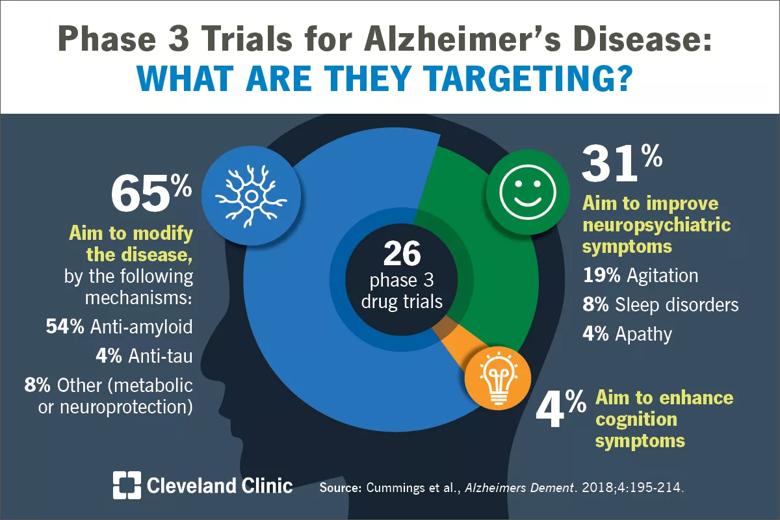
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d87ceac2-6295-46ba-997c-0cf2f6a2de11/18-NEU-4874-Alzheimers-Drugs-Visual-Abstract-2_jpg)
“Cleveland Clinic continues to be a leader in assessing the evolution of new therapies for Alzheimer’s disease, and we believe it’s crucial to drive conversation about our annual findings in an effort to foster collaboration and bring public awareness to the need for accelerated drug development,” says analysis co-author Aaron Ritter, MD, Director of the Clinical Trials Program at Cleveland Clinic Lou Ruvo Center for Brain Health.
The paper’s key observations include the following:
Advertisement
The authors note that proactive steps toward greater clinical trial efficiency are underway, including the widespread use of online registries to help recruit qualified trial participants. They suggest that optimizing the use of registries can help accelerate clinical trial execution and drug development, as can more-rapid clinical trial startup, pre-certified raters and use of a single review board.
The complete open-access article is available here.
Advertisement
Advertisement

Researchers explore the mental and physical benefits of social prescribing

Multidisciplinary approach helps address clinical and psychosocial challenges in geriatric care

Effective screening, advanced treatments can help preserve quality of life

Study suggests inconsistencies in the emergency department evaluation of geriatric patients

Auditory hallucinations lead to unusual diagnosis

How providers can help prevent and address this under-reported form of abuse

How providers can help older adults protect their assets and personal agency

Recognizing the subtle but destructive signs of psychological abuse in geriatric patients