Study shows linkage between weight loss amount and degree of benefit
Bariatric surgery, the most effective current treatment for patients with obesity, can significantly reduce many adult patients’ risks of developing and dying from obesity-related cancers, a new study led by Cleveland Clinic researchers has found.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Based on a median follow-up of 6.1 years, the SPLENDID (Surgical Procedures and Long-term Effectiveness in Neoplastic Disease Incidence and Death) matched-cohort study showed that, among adults with obesity, those who underwent bariatric surgery experienced a 32% lower incidence of cancer and a 48% lower incidence of mortality due to cancer than did patients in the nonsurgical control group. The findings held true in both subgroup and sensitivity analyses.
“Patients who undergo bariatric surgery typically lose 20% to 40% of their body weight and sustain it over decades,” says Ali Aminian, MD, the study’s lead author and Director of Cleveland Clinic’s Bariatric & Metabolic Institute. “What we found, which is striking, is that the greater the weight loss, the lower the risk of cancer.”
“According to the American Cancer Society, obesity is second only to tobacco as a preventable cause of cancer in the United States,” said the study’s senior author, Steven Nissen, MD, Chief Academic Officer of Cleveland Clinic’s Heart, Vascular & Thoracic Institute. “This study provides the best possible evidence of the value of intentional weight loss to reduce cancer risk and mortality.”
Numerous studies have shown the health benefits of bariatric surgery in patients with obesity. The Cleveland Clinic-led STAMPEDE study showed that following bariatric surgery, significant weight loss and control of type 2 diabetes were durable. The SPLENDOR study showed that in patients with fatty liver, bariatric surgery decreases the risk of the progression of liver disease to cirrhosis and serious heart complications.
Advertisement
The SPLENDID study, published in JAMA, adds important findings to the literature focused on the link between obesity and cancer. The study was named one of the 2023 Top 10 Clinical Research Achievements by Clinical Research Forum.
The worldwide prevalence of obesity is growing, with major negative impacts on public health. In the United States, nearly half of all adults have a body mass index of 30 or higher.
One consequence of obesity is excess cancer risk. Although the precise underpinnings of this linkage are unclear, obesity may influence tumor development or growth by triggering chronic inflammation, altering microbiota and increasing insulin resistance, circulating estrogens and adipokines.
Meaningfully evaluating the effects of intentional weight loss on cancer incidence and mortality is difficult because of the relatively low numbers of cancer cases among people with obesity, the challenges of sustaining significant weight loss using only lifestyle modification, and the long lag time between weight loss and cancer development.
Bariatric surgery’s success (coupled with lifestyle modifications) at achieving prolonged weight reduction provided an opportunity to assess potential impacts on cancer.
In SPLENDID, a group of 5,053 adult patients with obesity who underwent either Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) at Cleveland Clinic hospitals between 2004 and 2017 were matched 1:5 to a control group of 25,265 similar patients who did not undergo bariatric surgery. All had a body mass index (BMI) ≥35; median age was 46 years, 77% were female and 73% were white.
Advertisement
A logistic regression model was used for matching, which was performed based on 10 potential confounders, including demographic factors, BMI, smoking history, presence of type 2 diabetes, and Elixhauser and Charlson comorbidity indices.
The primary composite endpoint, estimated with multivariable Cox regression, was time to first incidence of one of 13 obesity-associated cancers: esophageal adenocarcinoma; renal cell carcinoma; postmenopausal breast cancer; cancer of the gastric cardia, colon, rectum, liver, gallbladder, pancreas, ovary, corpus uteri, or thyroid; and multiple myeloma. The secondary endpoint was cancer-related mortality.
“The cancers we looked at are strongly linked with obesity and we wanted to find out whether that risk was reversible with significant weight loss,” says Dr. Aminian. “We focused on bariatric surgery because it’s the most effective way to help patients with obesity lose weight.”
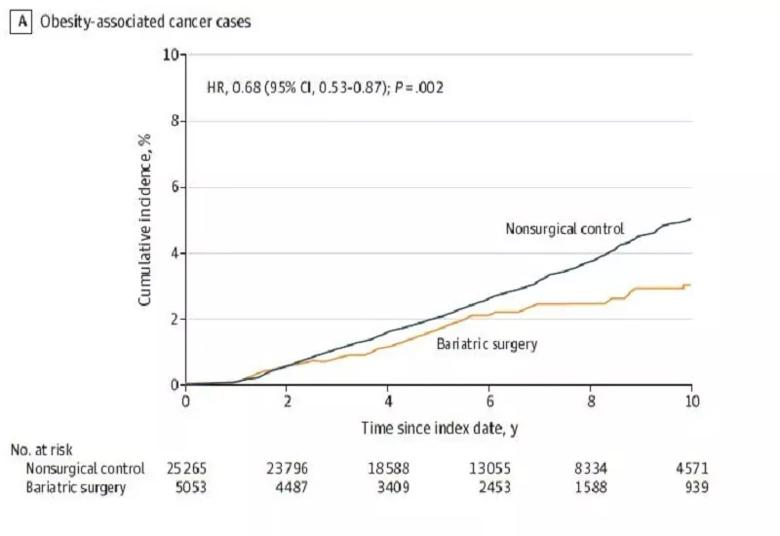
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8f02a1f4-e74d-4e4a-9f60-23fc6b43cd4a/22-DDI-2987571-JAMA-chart-800x550-1_jpg)
This figure shows the 10-year cumulative incidence estimates (Kaplan-Meier) for the primary endpoint: the first occurrence of 1 of 13 types of obesity-associated cancer. The median observation time was 5.9 years (IQR, 3.4-8.9 years) for patients in the bariatric surgery group and 6.1
years (IQR, 3.9-9.2 years) for patients in the nonsurgical control group.
Reproduced with permission from Aminian A, Wilson R, Al-Kurd A, et al. Association of Bariatric Surgery With Cancer Risk and Mortality in Adults With Obesity. JAMA. Published online June 03, 2022. © 2022 American Medical Association. All rights reserved.
Advertisement
At 10 years, the mean difference in body weight between the bariatric surgery groups and the nonsurgery group was 54.7 pounds (95% confidence interval [CI], 54.2-55.3 pounds) — a 19.2% (95%CI, 19.1%-19.4%) greater weight loss in the bariatric surgery group.
Ninety-six patients in the bariatric surgery group and 780 patients in the nonsurgical control group developed an obesity-associated cancer during the study’s 17-year follow-up period, which ended in 2021. Cumulative incidence of the primary endpoint of an obesity-associated cancer at 10 years was 2.9% (95% CI, 2.2%-3.6%) in the bariatric surgery group versus 4.9% (95% CI, 4.5%-5.3%) in the nonsurgical control group (absolute risk difference, 2.0% [95%CI, 1.2%-2.7%]; adjusted hazard ratio [HR], 0.68 [95%CI, 0.53-0.87], p = .002).
Twenty-one patients in the bariatric surgery group and 205 patients in the nonsurgery group died of cancer-related causes. Cumulative incidence of cancer-related mortality at 10 years was 0.8% (95% CI, 0.4%-1.2%) in the bariatric surgery group and 1.4% (95% CI, 1.1%-1.6%) in the nonsurgical group (absolute risk difference 0.6% [95% CI, 0.1%-1.0%]; adjusted HR 0.52 [95% CI, 0.31-0.88]; p=0.01).
Incidence of obesity-associated cancer was highest in the subgroup of surgical patients who lost <24% of their body weight. In the bariatric surgery group, increased weight loss was associated with further reduction of cancer risk in a dose-dependent relationship.
The two most common types of cancer that developed in the SPLENDID cohort were female breast cancer and endometrial cancer. Among all cancer types, endometrial cancer has the strongest association with obesity. The study found that bariatric surgery was associated with a significant reduction in endometrial cancer risk.
Advertisement
Across all of the different subgroups studied, the linkage between weight loss and reduction in cancer risk and cancer-related mortality was consistent, according to Dr. Aminian. “Whether patients were male or female, young or old, Black or white, or did or did not have diabetes, the risk reduction after surgically induced weight loss was consistent,” he says.
The SPLENDID researchers used multiple methods to evaluate the robustness of the association between bariatric surgery and cancer risk reduction. But they acknowledge some potential limitations, including the possibility of unmeasured confounders, the study cohort’s relatively young age (which could lead to a lower incidence of age-associated cancers), and the preponderance of Black and white participants (which could preclude generalizing the study’s findings to other racial or ethnic groups).
SG and RYGB are the two most common types of bariatric surgery. Although they differ in anatomical alterations and physiological effects, the two procedures result in relatively comparable weight loss outcomes. The Cleveland Clinic researchers posit, based on the overlap of Kaplan-Meier curves for RYGB and SG, that losing weight itself — not procedure-specific physiological changes related to alterations in anatomy — may be the principal mechanism for reduced risk of obesity-associated cancers.
“Further research needs to be done to understand the underlying mechanisms responsible for reduced cancer risk following bariatric surgery,” said Jame Abraham, MD, Chairman of the Department of Hematology and Medical Oncology in Cleveland Clinic’s Taussig Cancer Institute. “Based on the magnitude of benefit shown in our study, weight loss surgery can be considered in addition to other interventions that can help prevent cancer and reduce mortality.”
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors