Cohort celebrates pilot program success and takes a look ahead
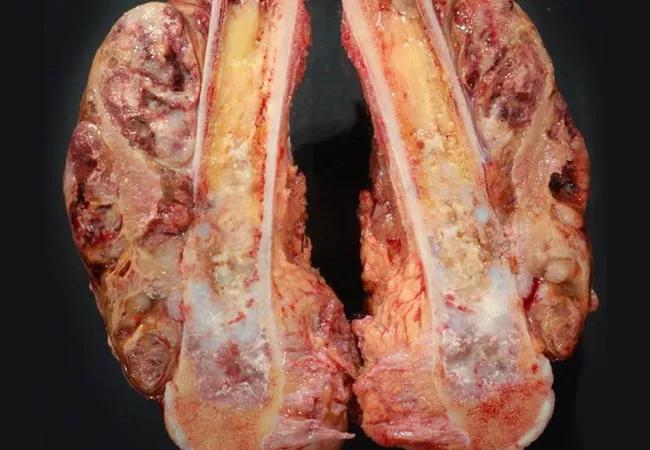
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/81c19e23-04f4-4dcb-a966-b419fc45a36b/20-ORI-1842192-Tumor-Registry-CQD_jpg)
20-ORI-1842192-Tumor-Registry-CQD
As the one-year pilot program of the Musculoskeletal Tumor Registry (MsTR), sponsored by the American Academy of Orthopaedic Surgeons (AAOS) concludes, participating healthcare institutions are now looking ahead to scale registry efforts and soon begin validating the data through research investigations.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The MsTR is a repository of longitudinal, outcomes-based data focused on rare bone and soft tissue tumors. This is the first musculoskeletal registry of its kind in the U.S., with collaboration from nationally renowned sarcoma tertiary care centers. Its surgeon leaders are hopeful it will power a new generation of research once the program is fully scaled.
Cleveland Clinic was one of six pilot sites that participated in 2019. The others included: Dartmouth-Hitchcock Medical Center, Johns Hopkins Medicine, Ohio State University, Stanford Health Care–Stanford Hospital and University of Iowa Hospitals and Clinics. In the first year, all sites were asked to collect patient demographics, patient and tumor baseline information, surgical details and postoperative data and patient-reported outcome measures. The sites recently met to share institutional experiences and best practices and agree on a final form of patient data to capture.
Nathan Mesko, MD, Center Director of Orthopaedic Oncology at Cleveland Clinic, remarks that the program covered much ground in its first year — from obtaining IRB approval, establishing programmatic expectations and streamlining workflow to the actual data collection and integration into the electronic medical record (Epic).
“We established foundational partnerships and critical programmatic pieces in the first year to begin building this robust data collection,” says Dr. Mesko. “We also really tried to address the overarching goals that the group was attempting to accomplish with this registry, in order to guide our thought process and data collection methodology for the future.”
Advertisement
“The goal was really to maximize the gain of information while minimizing the amount of effort required to put it in,” remarks Lukas Nystrom, MD, orthopaedic oncologist at Cleveland Clinic and programmatic leader for pediatric musculoskeletal oncology. “We understand that if this is going to be meaningful and effective for us as a group of providers, it has to be feasible and has to be integrated into our daily workflow,” he says.
The next step, the surgeon leaders say, is enrolling additional sites beyond the six pilot institutions. The registry will be open to anybody who wants to participate and has the technical capabilities to do so.
The team plans to then begin validating the utility of the data in the registry. Research efforts are underway immediately and will likely come to fruition in the fall of 2020.
“As you can imagine, with rare tumors, it can take a very long time to collect enough data to attain statistically significant power that will allow us to answer questions that can be confidently used to change practice. Collecting data prospectively enables us to ask questions ahead of time, while being be more efficient and sophisticated in our approach toward answering them,” says Dr. Mesko.
This prospective registry will allow sarcoma researchers to revisit older literature, ask the same questions using new data with larger numbers — and ask altogether new questions — to really investigate the utility of this prospective data and its capacity to shape new paradigms in care.
Advertisement
Longer-term goals for the MsTR include expanding the registry to include broad national representation in the next five to 10 years. They also hope to establish an institutional tissue bank linked to their internal registry, which Drs. Mesko and Nystrom note would create new opportunities for basic and translational investigations.
The surgeons agree that the registry is an enormous step closer to aggregating data around a specific diagnosis and treatment options in a very short amount of time. “The world of sarcoma is at our finger tips in terms of research questions and capabilities,” says Dr. Nystrom. “I think this is just going to open up many, many doors for us.”
Advertisement
Advertisement
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches
Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer