First step toward testing therapeutics
By Michael Ramos; Alex Yuan, MD, PhD; and Arun Singh, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A patient is diagnosed with uveal melanoma. Thanks to therapeutic advances, enucleation is now a last resort. A multidisciplinary team of ophthalmologists and radiation oncologists develops a plan to treat the patient with targeted radiotherapy. They elect to use a radioactive plaque, a treatment known as brachytherapy, to deliver high-dose radiation to the tumor.
The tumor shrinks significantly. However, the patient develops the serious vision-impairing complication of radiation retinopathy.
Radiation retinopathy is a broad term describing a spectrum of retinal changes following radiation exposure.
Classically defined by its vasculopathy, radiation retinopathy typically develops six months to three years following irradiation. It begins with preferential loss of endothelial cells, leading to vessel occlusion, leakage and retinal nonperfusion. As the disease progresses, retinal layers are compromised, further impairing vision. Late-stage radiation retinopathy is characterized by ischemia-induced ocular neovascularization.
Despite an established disease progression, the underlying pathophysiological mechanisms of radiation retinopathy remain unclear.
Treatments include risk-factor modification, such as:
Modest success has been achieved with these therapies. However, they fail to address the cellular and molecular events leading to radiation retinopathy, and prevention strategies remain limited.
Advertisement
The lack of elucidated mechanisms and dedicated treatment options demonstrates a clear need for more robust research. Similar to other retinal vasculopathies such as diabetic retinopathy, radiation retinopathy research can benefit from the mindful use of an appropriate animal model — a useful tool in the quest to understand disease pathologies.
Despite its near 95% effectiveness and use as a first-line treatment in many cancers, episcleral plaque brachytherapy is commonly associated with radiation retinopathy.
Because no brachytherapy-induced radiation retinopathy animal model exists, we sought to establish one. It is our goal to set the stage for more mechanistic studies and eventually test promising therapeutics in a model closest to clinical experience.
Several factors need to be considered when establishing a radiation retinopathy model:
Advertisement
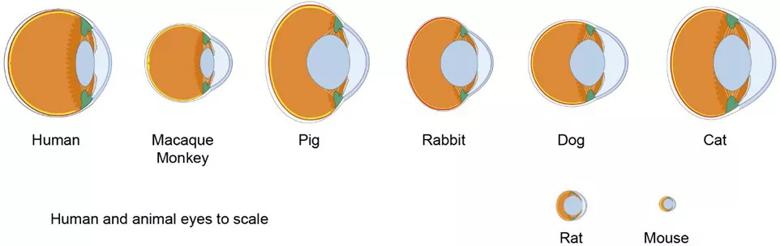
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fbcebb0a-475f-4ea1-b877-3cfa19b7eeb3/19-EYE-4260-Animal-Research-CQD-Inset-1_jpg)
Figure 1. Differences in ocular anatomy among various species must be considered when creating a model of radiation retinopathy.
Image from Ramos MS, Echegaray JJ, Kuhn-Asif S, et al. Animal models of radiation retinopathy – From teletherapy to brachytherapy. Exp Eye Res. 2019 Apr;181:240-251. Copyright 2019, with permission from Elsevir.
To create our model, a 1 mm by 4 mm radioactive iodine-125 seed was surgically implanted posterior to the limbus of the left eye of Lewis rats (Figure 2). The initial dose of radiation treatment lasted six hours, after which the seed was removed. A total dose of 45 Gy at a distance of 1 mm from the seed was delivered. Escalating dosages of radiation will be administered to find the optimal range.
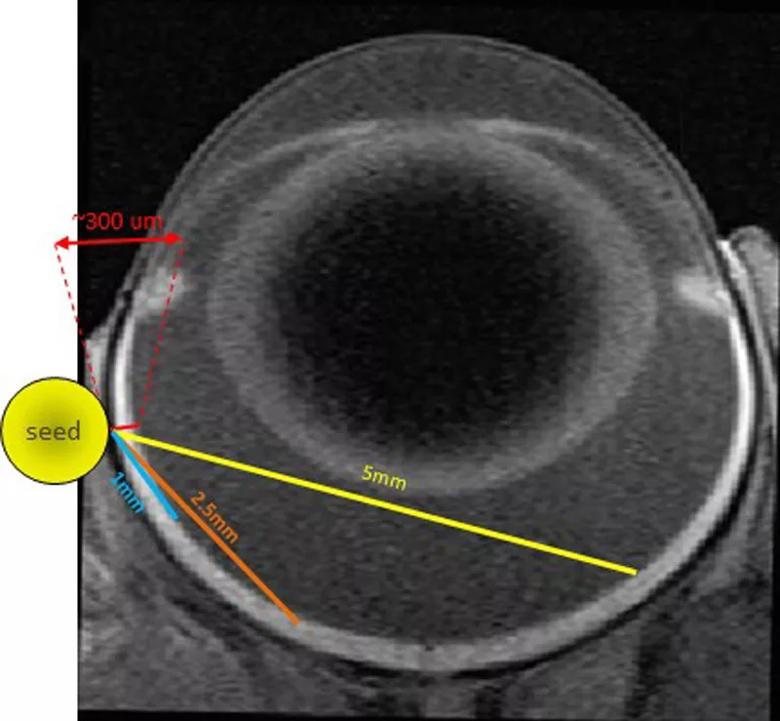
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/0a35d1b7-3d79-47d5-a9f2-d78871b783b2/19-EYE-4260-Animal-Research-CQD-Inset-2_jpg)
Figure 2. Placement of a 1 mm x 4 mm radioactive Iodine-125 seed surgically implanted posterior to the limbus of the left eye of a Lewis rat.
For 12 months, rats will be followed using optical coherence tomography (OCT) and wide-field fluorescein angiography (FA) to monitor the appearance of retinopathy (Figure 3). Based on previous reports using external beam radiation, most animals show signs of retinopathy (i.e., dot hemorrhages, cotton wool spots or retinal thinning) approximately six months after treatment.
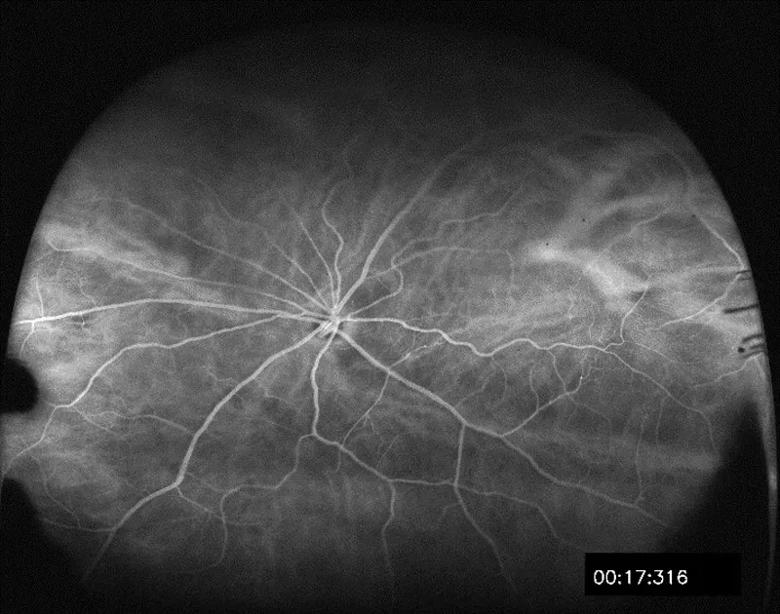
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/77626ff7-344f-4fc0-9506-79d00fb29c9b/19-EYE-4260-Animal-Research-CQD-Inset-3_jpg)
Figure 3. An example of wide field fluorescein angiography imagery used to monitor the development of radiation-induced retinopathy in a Lewis rat.
Moving forward
This pilot study is the first attempt at episcleral plaque brachytherapy-induced radiation retinopathy in an animal model. In conjunction with the appropriate model, clinically relevant imaging modalities, such as OCT and wide-field FA, will allow for easier in vivo comparative anatomical assessment and classification of radiation-induced retinopathic changes.
Advertisement
Future investigations can dive deeper into potential pathophysiological mechanisms, such as those involving inflammatory pathways, leukocytes, microglia and apoptosis. These studies will allow for a better understanding of radiation retinopathy and the development of more effective therapies of this disease.
Mr. Ramos is a research technician at Cole Eye Institute. Dr. Yuan is a retina specialist. Dr. Singh is Director of the Department of Ophthalmic Oncology.
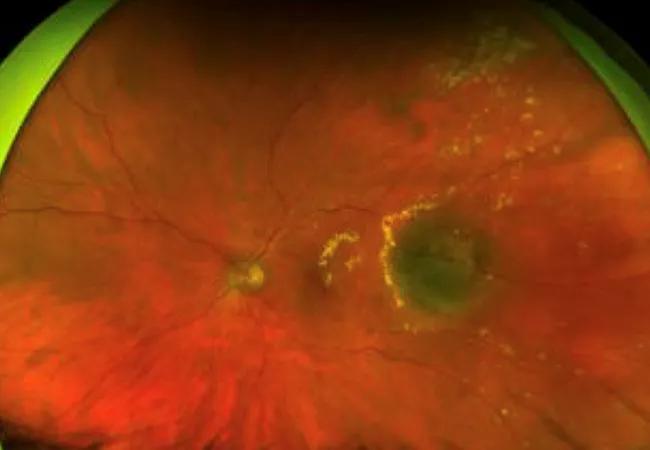
Feature image: A color fundus photo of the clinical presentation of a left eye affected by radiation retinopathy shows multiple retinal exudates (yellow material) surrounding the irradiated tumor.
Image from Ramos MS, Echegaray JJ, Kuhn-Asif S, et al. Animal models of radiation retinopathy – From teletherapy to brachytherapy. Exp Eye Res. 2019 Apr;181:240-251. Copyright 2019, with permission from Elsevir.
Advertisement
Advertisement

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists