Key findings from national database study
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Outcomes in pediatric patients with acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) have improved in more recent eras. Advancements in disease-directed therapy as well as supportive care have decreased the rate of relapse and treatment-related mortality.1 Yet, despite improvements in treatment for children and adolescents and young adults (AYAs; age range 15-39 years) with acute leukemia, certain health services issues may affect outcomes.
We hypothesized that distance to treatment center affects outcomes of children and young adults with acute leukemia because a longer distance might strain families and caregivers to a greater degree and might lead to delays in seeking care both at diagnosis and for complications during treatment.2
To test our hypothesis, we queried the National Cancer Database and developed models to predict the contribution of distance to treatment center to overall survival for children and AYAs with AML and ALL. In our analysis, we included patients 39 years old or younger who were diagnosed with AML or ALL between 2004 and 2015.
Distance to the treatment center was determined by measuring the cancer center’s address to the center of the zip code of the patient’s address and was categorized as: < 10 miles, > 10-≤ 20 miles, > 20-≤ 50 and > 50 miles. Other factors that were analyzed included Carleson-Deyo comorbidity score, sex, race/ ethnicity, insurance status, community median income, year of diagnosis, age, and rural vs. urban zip code. Models of overall survival were stratified based on the type of leukemia.
Advertisement
In total, 12,301 patients with AML and 22,683 patients with ALL were analyzed (Figure 1).
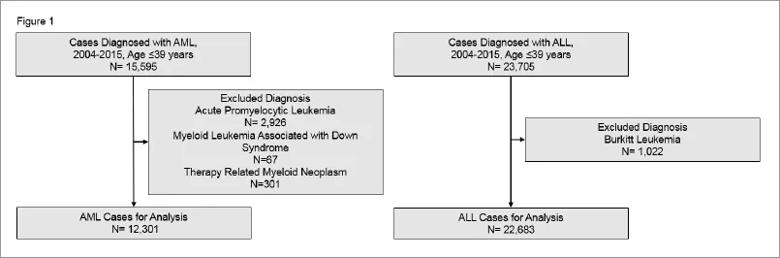
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/30f5d059-4150-4241-b144-a4c942e44058/Rotz-Chart_jpg)
The final ALL model included distance to treatment center, Charlson-Deyo score, age, race, insurance status, and community income level. Compared with distances > 50 miles, all other distances were associated with improved survival (hazard ratio [HR] for ≤10 miles, 0.91; P = .04; HR for > 10 to ≤ 20 miles, 0.86; P = .004; HR for > 20 to ≤ 50 miles, 0.87; P = .005). The final model for AML included the same variables as the ALL model, except for distance to treatment center, which was not statistically significant.
Interestingly, for patients with ALL, the distance to treatment center remained significant after adjustments for other factors such as community income and insurance status. Furthermore, rural and urban zip codes were not statistically significantly different in this study. Taken together, these data suggest that the physical distance to the treatment center, as opposed to only the intrinsic community factors, influences overall survival for ALL patients.
The association between a distance to treatment center > 50 miles and poorer overall survival for both children and young adults with ALL—but not AML—is intriguing. Unfortunately, data from the NCDB do not discern between treatment-related and disease-related mortality; however, it is plausible that the difference in overall treatment approaches for ALL and AML may be responsible.
The typical treatment for ALL is delivered mostly on an outpatient basis and extends for multiple years. ALL treatment requires patients to take many trips to the treating center for therapy and intermittent complications (infections, dehydration, etc.). Conversely, AML treatment is typically given almost exclusively on an inpatient basis and lasts for months rather than years.
Advertisement
We surmise that potential challenges for patients with ALL related to traveling to appointments and leading to delays in therapy or difficulty in getting to the treatment center for emergent complications might be responsible for the differences observed in this study. We conclude that increased attention to adherence, supportive care and logistics for patients with ALL traveling long distances is warranted.
References
About the author: Dr. Rotz is Assistant Professor of Pediatrics, Cleveland Clinic Lerner College of Medicine at Case Western Reserve University.
Advertisement
Advertisement

Findings hold lessons for future pandemics

One pediatric urologist’s quest to improve the status quo

Overcoming barriers to implementing clinical trials

Interim results of RUBY study also indicate improved physical function and quality of life

Innovative hardware and AI algorithms aim to detect cardiovascular decline sooner

The benefits of this emerging surgical technology

Integrated care model reduces length of stay, improves outpatient pain management

A closer look at the impact on procedures and patient outcomes