1-Minute Consult: Updated recommendation reflects reduced incidence of disease
By Craig D. Nielsen, MD, FACP and Xian Wen Jin, MD, PhD, FACP
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The short answer is no. In the summer of 2019, the U.S. Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (ACIP) removed its 2014 recommendation that all healthy adults 65 or older should receive the PCV13 vaccine followed in one year by the PPSV23 (23-valent pneumococcal polysaccharide)
vaccine.1
However, PCV13 can still be given after engaging in shared clinical decision-making with the patient. The ACIP continues to recommend that all patients in this population receive the PPSV23 vaccine.1,2
In its 2014 recommendation for pneumococcal vaccination in all adults 65 and older, the ACIP noted that certain high-risk groups should be vaccinated earlier or receive additional doses.1 Pallotta and Rehm outlined these recommendations and discussed the rationale for vaccinating against Streptococcus pneumoniae to prevent invasive pneumococcal disease.3 So why did the ACIP modify its recommendation?
The primary reason is that the incidence of PCV13-type pneumococcal disease in adults had gone down to historic lows (Figure 1). A key to this reduction was that children started to be vaccinated in 2000, at first with PCV7 and then with PCV13, which replaced PCV7 in 2010.1,2 This incidence leveled out from 2014 to 2018 despite the ACIP recommendation and data showing that about half the Medicare beneficiaries older than 65 received the vaccine.4 The decreased incidence of disease also has decreased the cost-effectiveness of vaccination. The cost of giving PCV13 and then PPSV23, compared with PPSV23 alone, is now estimated to be $200,000 to $560,000 per quality-adjusted life year,1 whereas in 2014 it was only $65,000.5
In light of these facts, the ACIP voted to remove the recommendation requiring older patients to receive the PCV13 vaccine.
Advertisement
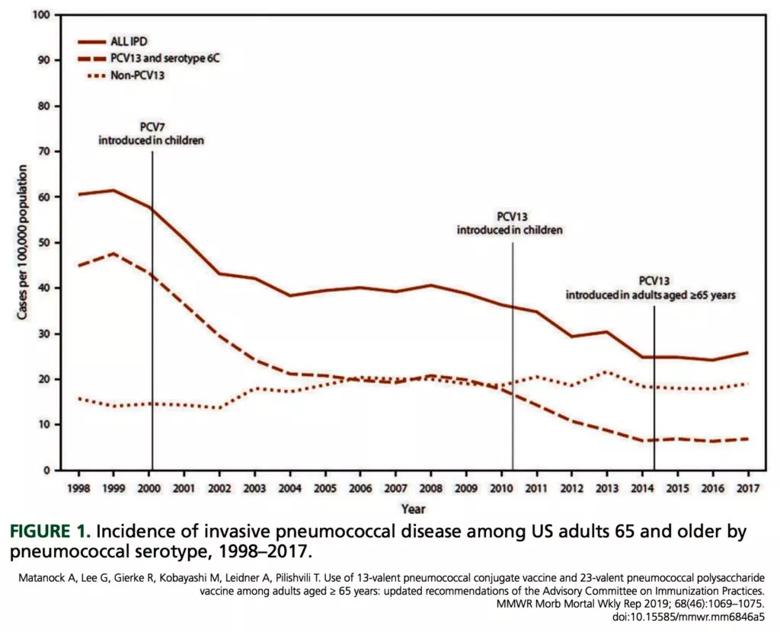
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6b3f67e4-4124-4d5b-9897-82536313926c/Incidence-of-invasive-pneum-1024x830_jpg)
But it is not that simple. In an attempt to balance “the minimal population-level impact of routine [vaccination] with the potential for individual-level protection,”1 the ACIP added the principle of shared clinical decision-making to its recommendation.1,2,4 The ACIP committee recognized that some immunocompetent patients at higher risk of invasive pneumococcal disease (or their physicians) may believe that PCV13 would still be worthwhile. This population includes patients with certain medical conditions (eg., alcohol or tobacco abuse; chronic heart, liver, or lung disease; diabetes) and patients living in nursing homes or other long-term care facilities.1,4 By adding the concept of shared clinical decision making, the ACIP committee ensured that “PCV13 would remain available to patients who want this added protection.”1
A practical downside of this recommendation is that busy primary care practitioners may lack the time to effectively and efficiently review the potential benefit or lack of benefit of PCV13 for the individual. Also, universal recommendations (eg., vaccinate all patients over age 65) are generally easier to remember or implement than conditional recommendations (eg., vaccinate some patients over age 65). Strategies known to improve vaccination compliance rates include interventions such as electronic medical record reminders and direct patient outreach.
The 2019 ACIP recommendations are to vaccinate all patients age 65 and older with PPSV23, but PCV13 can be used in shared clinical decision-making. The ACIP continues to endorse use of both PCV13 and PPSV23 in patients older than 19 (including those 65 and older) with immunocompromising conditions, cerebrospinal fluid leak or cochlear implants.1 These recommendations are summarized in Table 1.2
Advertisement
1. Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;
68(46):1069–1075. doi:10.15585/mmwr.mm6846a5
2. Freedman M, Kroger A, Hunter P, Ault KA; Advisory Committee on Immunization Practices. Recommended adult immunization schedule, United States, 2020. Ann Intern Med. 2020; 172(5):337–347.
doi:10.7326/M20-0046
3. Pallotta A, Rehm SJ. Navigating pneumococcal vaccination in adults. Cleve Clin J Med 2016; 83(6):427–433.
doi:10.3949/ccjm.83a.15044
4. Shah AA, Wallace MR, Fields H. Shared decision-making for administering PCV13 in older adults. Am Fam Physician 2020; 101(3):134–135. pmid:32003958
5. Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016; 31(8):901–908. doi:10.1007/s11606-016-3651-0 Address: Craig D. Nielsen, MD, FACP, Department of Internal Medicine,
G10, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195;
nielsec@ccf.org
Note: This article was originally published in the Cleveland Clinic Journal of Medicine.
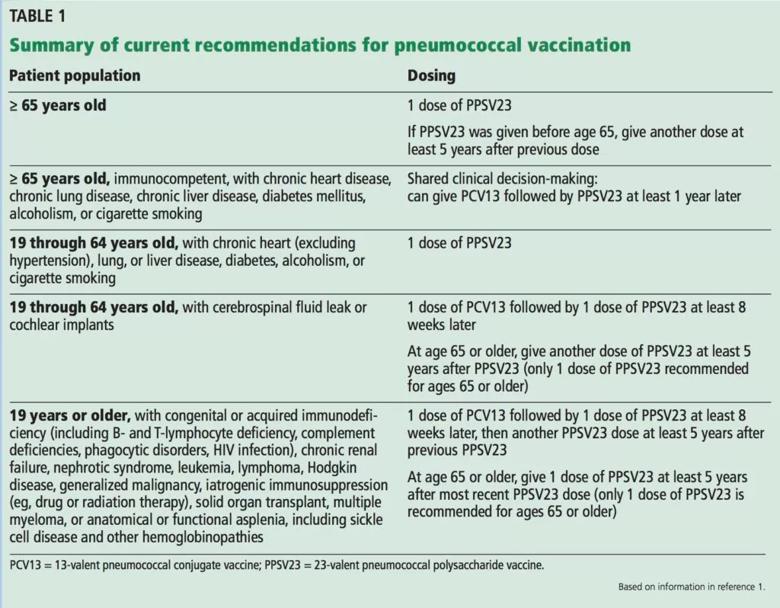
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/73d0ffb4-583b-44b1-8e8b-d2eb574e231a/Recommendations-for-Pneumococcal-Vx-1024x798_jpg)
Advertisement
Advertisement

Researchers explore the mental and physical benefits of social prescribing

Multidisciplinary approach helps address clinical and psychosocial challenges in geriatric care

Effective screening, advanced treatments can help preserve quality of life

Study suggests inconsistencies in the emergency department evaluation of geriatric patients

Auditory hallucinations lead to unusual diagnosis

How providers can help prevent and address this under-reported form of abuse

How providers can help older adults protect their assets and personal agency

Recognizing the subtle but destructive signs of psychological abuse in geriatric patients