Case reports of 2 single-patient protocols
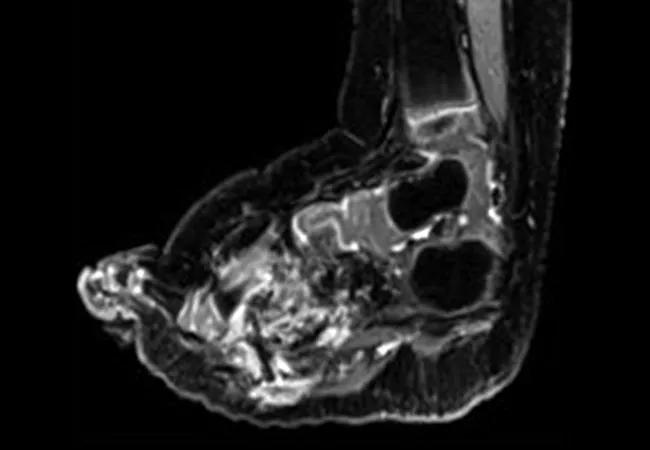
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/79acf6e3-7f43-4e6f-84f7-e5a2a50b8f36/650x450-Selpercatinib-in-Pediatric-Patients-with-RET-Altered-Tumors_jpg)
650×450-Selpercatinib-in-Pediatric-Patients-with-RET-Altered-Tumors
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
The RET gene can be oncogenically activated by point mutations, in-frame deletions and chromosomal rearrangements,1,2 gain-of-function events that render the RET tyrosine kinase constitutively active.3-5 In pediatric and young adult patients, RET gene fusions have been reported in 22% to 45% of papillary thyroid carcinomas (PTCs)6-9 and less frequently in pediatric and young adult patients with glioma,10 lipofibromatosis,11 inflammatory myofibroblastic tumor,12 and infantile myofibromatosis.13 In addition, activating point mutations of RET have been reported in 40% to 50% of sporadic medullary thyroid cancers (MTCs).14,15 If they are constitutional, such mutations lead to the hereditary autosomal-dominant cancer syndrome called multiple endocrine neoplasia type 2 (MEN2), one characteristic of which is predisposition to MTC.16,17 Currently, no highly specific RET-targeted agents are approved for the treatment of patients with RET-altered cancers.
Selpercatinib (LOXO-292) is a potent, adenosine triphosphate-competitive, highly selective small molecule RET inhibitor with nanomolar potency against varied RET alterations (including anticipated acquired gatekeeper resistance mutations).18,19 Preliminary results for selpercatinib in a phase I/II trial (LIBRETTO-001; ClinicalTrials.gov identifier: NCT03157128) are highly encouraging, showing that it is generally well tolerated and has marked antitumor activity in adolescent and adult patients with RET-altered cancers, including those with brain metastases and those with tumors resistant to previous multitargeted kinase inhibitors.20,21
Advertisement
Given the lack of other treatment options, access to selpercatinib for the four patients ineligible for an ongoing clinical trial was enabled by single-patient protocols that were allowed by country-specific regulatory agencies and approved by institutional review boards. Two cases from the original publication are shared below. These patients received selpercatinib (capsule or liquid formulation) orally in continuous 28-day cycles at a starting dose of 90 mg/m2 twice per day. This dose was intended to deliver exposure equivalent to the recommended adult phase II dose of 160 mg twice per day.
A 7-year-old boy with neonatal hypotonia, abdominal pain, hollow feet, and unexplained laryngeal spasms was referred for diagnostic exome sequencing of genomic DNA (SureSelect XT Clinical Research Exome, Agilent, Santa Clara, CA). This revealed a constitutional de novo RET M918T mutation, a pathogenic variant associated with multiple endocrine neoplasia type 2 (MEN2) that confers the highest risk of early onset (medullary thyroid cancer (MTC).16 The patient was subsequently diagnosed with an MTC and underwent thyroidectomy with tracheostomy followed by vandetanib therapy, which was discontinued because of the onset of grade 3 colitis.
Selpercatinib was subsequently initiated at 90 mg twice per day. Treatment-related adverse events included grade 1 vomiting and diarrhea. After two cycles of treatment, stable disease was observed.
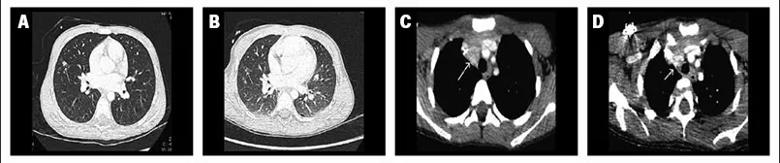
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/cbc927ad-e876-40c8-8133-54ee0ec279b5/805x-INSET-1-Selpercatinib-in-Pediatric-Patients-with-RET-Altered-Tumors_jpg)
Caption: Computed tomography scans at baseline and after four months of treatment with selpercatinib of thorax (A, B, respectively) and mediastinum (C, D, respectively) showing metastatic disease in a patient with RET-mutated medullary thyroid cancer. Early disease control was achieved after two cycles of treatment.
Advertisement
An otherwise healthy 21-month-old girl had been diagnosed with lipofibromatosis of her left foot at birth. This had progressively increased in size and affected her ability to ambulate. She was evaluated by oncologists and surgeons who recommended amputation. Next generation sequencing analysis of a biopsy specimen (FoundationOne CDx; Foundation Medicine, Cambridge, MA) identified an NCOA4-RET fusion, which was confirmed by whole genome and transcriptome analysis. Selpercatinib was initiated, and imaging after two months revealed a partial response by RECIST 1.1, with a 59% reduction in tumor volume and resolution of tumor infiltration of the metatarsals (Fig 2I-L). No adverse events were reported.
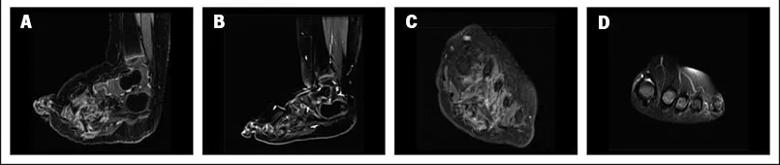
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/037a2ff6-9d9f-416c-9db4-992cba8eaef5/805x-INSET-2-Selpercatinib-in-Pediatric-Patients-with-RET-Altered-Tumors_jpg)
Caption: CT scans (A, B) at baseline and (C, D) after two months of treatment with selpercatinib of the left foot in a patient with an NCOA4-RET fusion–positive lipofibromatosis. Selpercatinib treatment resulted in a significant decrease in tumor burden leading to improvements in gait and locomotion.
RET alterations are actionable oncogenic drivers that occur commonly in MTCs,24 pediatric papillary thyroid carcinomas (PTCs),6-9 and rarely in other pediatric cancers.10-13,25 The multitargeted kinase inhibitors cabozantinib, vandetanib and lenvatinib have demonstrated modest antitumor activity in adult and pediatric patients with MTC,26-29 and adult patients with RET fusion-positive cancers.4,30,31 The clinical activity of these agents is limited by suboptimal RET inhibition and significant toxicity, most likely because of strong inhibition of other kinases such as KDR and VEGFR2.5,32
Advertisement
Selpercatinib has demonstrated durable tumor responses and high tolerability in adolescents and adults with RET-altered cancers, with response rates of 68% reported in patients with RET fusion-positive platinum-pretreated non-small-cell lung cancer,20 and 56% in patients with RET mutation-positive cabozantinib and/or vandetanib pretreated MTC.21 The results described here in patients with limited treatment options indicate that selpercatinib is also effective and safe in pediatric patients whose tumors harbor RET alterations. A phase I/II pediatric trial for patients with advanced RET-altered solid or primary CNS tumors is ongoing (LIBRETTO-121; ClinicalTrials.gov identifier: NCT03899792).
*This abridged article is published under the Creative Commons Attribution 4.0. To see the unabridged version, including a complete set of references, please see: Ortiz MV, Gerdemann U, Govinda S, et al. Activity of the highly specific RET inhibitor selpercatinib (LOXO-292) in pediatric patients with tumors harboring RET gene alterations. JCO Precision Oncology. 2020 Apr:4;341-347.
Advertisement
Advertisement
Goal-of-care discussions drive earlier hospice access
Clinical trials and de-escalation strategies
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting