New phase 2 data boost hopes for phase 3 Lp(a) HORIZON trial
Lipoprotein(a), or Lp(a), is a distinctive particle with two components: a lipoprotein core that resembles LDL, along with a shell that contains apolipoprotein(a), or apo(a). It’s also distinctive for having been dubbed one of the final frontiers in lipid management — and for good reason. Elevated blood Lp(a) levels are primarily due to genetic variations in the LPA gene that encodes for apo(a) and cannot be lowered by diet, exercise or current lipid-lowering therapies.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“By combining the atherosclerotic effects of LDL with the prothrombotic effects of apo(a), elevated Lp(a) essentially delivers a double whammy of noxious atherothrombotic effects to affected individuals,” explains Steven Nissen, MD, Chief Academic Officer of Cleveland Clinic’s Heart & Vascular Institute.
Those effects manifest as a heightened risk — and often an accelerated course — of cardiovascular diseases, most notably premature myocardial infarction (MI), venous thromboembolism and calcific aortic stenosis.
Interestingly, despite the progress in reducing LDL-C over the past three decades, there remains a subset of patients whose LDL-C levels do not fall as expected after optimal therapy with statins or other lipid-lowering medications. In some of these cases of so-called statin resistance, the culprit is a very high level of Lp(a), which contributes to the laboratory-measured levels of LDL-C.
Normal Lp(a) levels are considered to be less than 25 mg/dL, with significant risk of atherothrombotic events beginning at levels between 50 and 70 mg/dL and rising thereafter. And that risk is not at all rare: 64 million U.S. residents have an Lp(a) level of 60 mg/dL or higher. Over 3 million have levels of 180 mg/dL or more, which confer extremely high risks.
Despite such widespread and significant clinical stakes, Lp(a) levels are infrequently measured in clinical practice, chiefly because there have been no effective Lp(a)-lowering pharmacotherapies to date. That includes statins, which actually can slightly raise Lp(a) levels.
Advertisement
This absence of therapies is likely to soon end, however, thanks to one or more gene silencing approaches to Lp(a) reduction now under investigation.
The approach that’s furthest along involves antisense oligonucleotide (ASO) therapy and is the focus of a newly launched international phase 3 trial with Cleveland Clinic as the coordinating center, Dr. Nissen as study chairman and Cleveland Clinic Co-Section Head of Preventive Cardiology, Leslie Cho, MD, as a principal investigator.
“The ASO approach to gene silencing involves single-stranded DNA that binds to messenger RNA, which is subsequently degraded so that the message to produce apo(a) never gets transmitted,” explains Dr. Nissen (see figure). “It’s as if you’re turning off the LPA gene responsible for elevated Lp(a) levels.”
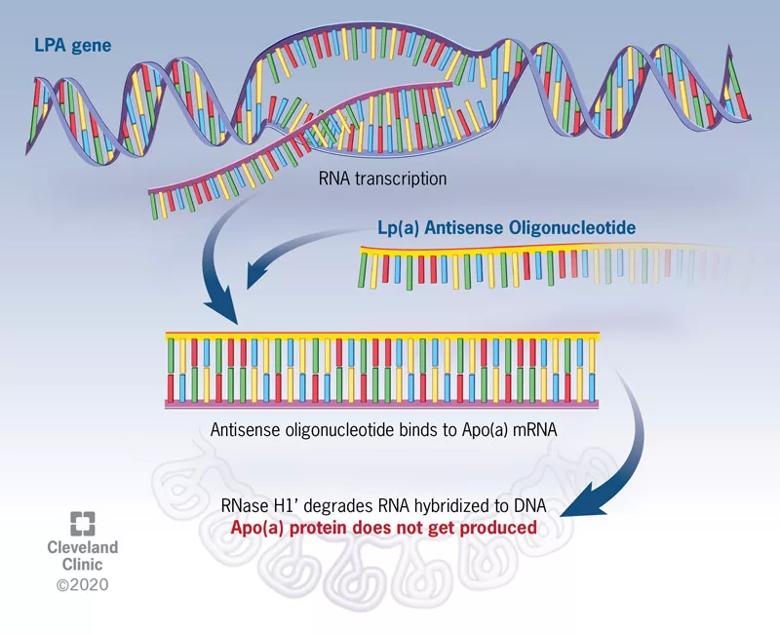
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7bf19d7b-e397-4ec7-accd-57d7f9e50ca6/20-HRT-001-LPa-CQD-Inset_jpg)
Figure. Mechanism of action of Lp(a) antisense therapy. The antisense oligonucleotide (ASO) is a DNA-like strand that combines with the messenger RNA responsible for production of apolipoprotein(a), or Apo(a). The complex of Apo(a) and the ASO is subsequently degraded by RNases. In the absence of Apo(a), the Lp(a) particle cannot be assembled, and circulating levels fall by up to 80%.
He notes that the specialized ASO therapy, known as APO(a)-LRx, is conjugated with N-acetyl-galactosamine (GalNAc3), an efficient ligand for the asialoglycoprotein receptor on the surface of hepatocytes. “This approach helps the therapy accumulate in the liver so that it doesn’t reside much in the circulation, which minimizes potential adverse effects,” he says.
Advertisement
Conjugation with GalNAc3 increased the therapy’s potency up to 30-fold over that of the parent ASO, allowing much lower dosing and improved tolerability. In newly published phase 2 trial data in 286 patients (N Engl J Med. 2020;382:244-255), 20 mg of APO(a)-LRx once weekly reduced plasma Lp(a) levels by a mean of 80% with no notable safety issues.
The new phase 3 trial that Dr. Nissen is chairing, known as Lp(a) HORIZON (NCT04043552), aims to definitively assess the efficacy and safety of APO(a)-LRx among 7,680 patients worldwide. Participants will have established coronary artery disease and fall into one of two Lp(a) strata: ≥70 mg/dL and ≥90 mg/dL. They will receive optimal background therapy, including statins, and be randomized to four years of therapy with either placebo or APO(a)-LRx 80 mg given by subcutaneous injection once monthly.
Lp(a) HORIZON is an outcomes trial, with the primary measure being time to first occurrence of the composite endpoint of cardiovascular death, nonfatal MI, nonfatal stroke or urgent coronary revascularization requiring hospitalization. Outcomes will be evaluated for both strata of baseline Lp(a) levels (≥70 and ≥90 mg/dL). Study completion is expected in 2024.
“This is a trial with enormous public health implications,” notes Dr. Nissen. “Lp(a) reduction does indeed represent one of the last frontiers in lipid management. We are optimistic as this investigation gets underway.”
Image at top is based, in part, on a model created using Molecular Maya (https://clarafi.com/tools/mmaya/) and PDB ID 1JFN (Maderegger B, Bermel W, Hrzenjak A, Kostner GM, Sterk H. Solution structure of human apolipoprotein(a) kringle IV type 6. Biochemistry. 2002;41:660-668.).
Advertisement
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more