Imaging aids selection of embryos for uterine transfer
By Nina Desai, PhD, HCLD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In vitro fertilization (IVF) success rates have increased since the birth of the first IVF baby in 1978. However, in 2012 the implantation rate per embryo transferred in patients younger than 35 was still only 37.5 percent, according to the Society for Assisted Reproductive Technologies national IVF registry.
To maximize the chance of pregnancy, centers typically transfer two to three embryos per cycle, despite the increased risk of multiple pregnancy and its associated neonatal and maternal complications. The most critical step during IVF is embryo selection for transfer.
For the last two decades, this selection has been based solely on morphology. Currently, these morphological assessments are limited to once a day, since repeated removal of the embryo culture dish from the incubator environment may result in exposure of embryos to undesired temperature and pH shifts. Often, embryo morphology is very similar, making selection difficult (Figure 1).
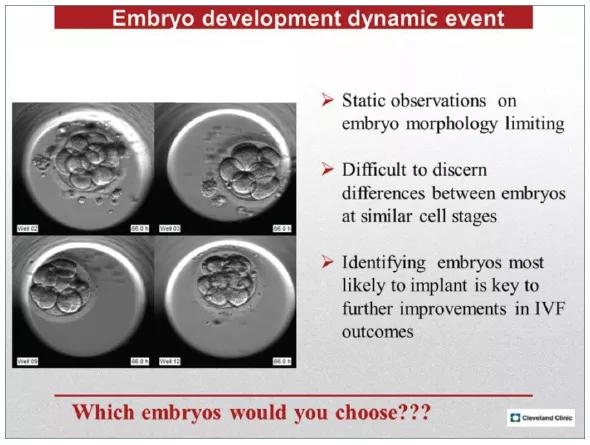
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fbbecd46-9889-4944-a924-bf1edcdd8d93/figure11_jpg)
Figure 1. Micrograph depicts four embryos on day 3 of culture. With conventional culture, the embryologist typically selects two embryos for transfer based strictly on morphology. Only two of the four embryos shown will develop to blastocysts with the ability to implant (wells 2, 9) while the remaining two will arrest in culture. Time-lapse imaging offers additional data on the timing of early cleavages, synchronicity of cell divisions, and the presence of cleavage or nuclear anomalies, which could aid in distinguishing among embryos of similar morphology and ultimately in selecting embryos with the greatest implantation potential.
Advertisement
Improvements in embryo selection for transfer could help reduce overall multiple pregnancy rates after IVF by allowing the transfer of fewer embryos without compromising the patient’s opportunity to achieve a pregnancy.
The introduction of time-lapse imaging systems in to clinical IVF laboratories has enabled more detailed observations on embryo developmental kinetics. It has become clear that embryo development is a dynamic event and static observations of embryonic growth can be limiting in their ability to discern differences between embryos at similar cell stages.
Numerous data suggest that the precise timing of specific events such as pronuclear formation, syngamy, cell division, cell cycle intervals, synchronicity of cell division and initiation of blastocyst formation are indicators of an embryo’s developmental potential. The ability to continuously monitor an embryo’s progression toward these benchmarks may therefore aid selection of the best embryos for uterine transfer.
In 2012 Cleveland Clinic’s IVF laboratory acquired its first time-lapse imaging system, the EmbryoScope® (Unisense Fertilitech, Denmark). This elegantly engineered instrument is an incubator with a built-in microscope and high-definition camera, allowing continuous monitoring of embryo growth. The chamber design and camera software allow imaging of as many as 72 embryos (six patient slides with 12 embryos apiece) in five focal planes every 15 minutes.
After years of “quick peeks” at embryos once daily, being able to actually visualize the journey from fertilization through blastocyst formation to hatching was amazing. Very quickly we began to see early cleavage and nuclear anomalies not previously evident, and to understand the impact of these, as well as the timings of cell cycle events, on subsequent pregnancy outcomes.
Advertisement
Our first paper on EmbryoScope use and time-lapse imaging was recently published in Reproductive Biology and Endocrinology.1 This study presents our first experiences and initial clinical outcomes data with continuous time-lapse imaging in the Embryoscope. We analyzed morphokinetic data collected every 15 minutes from 648 embryos cultured in vitro for five days. Timing of early cell division of the zygote to two, four and eight cells as well as progression to morula and blastocyst stages were important. Cell cycle intervals and time to complete synchronous divisions also provided insight into the embryo’s potential.
The controlled culture environment that the EmbryoScope provided, allowing undisturbed growth with continuous imaging, positively impacted clinical results. Our clinical outcome data are shown in Table 1. The clinical pregnancy rate for day 5 transfers was 72% (41/57). The implantation rate per embryo transferred was 50% (61/121). This study allowed us to define the optimal timing for transition from a one-cell zygote to the blastocyst stage.
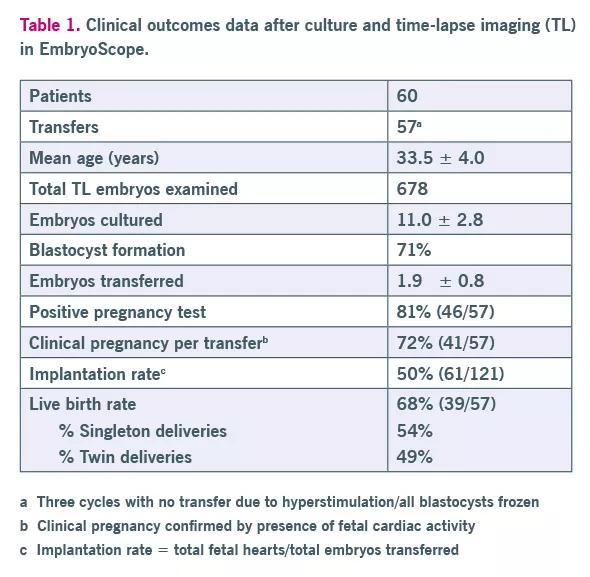
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a866a5c3-fa12-47b4-8cd4-6f859b0028c8/table1_jpg)
Table 1. Clinical outcome data.
The first three cleavage events transforming from one to two cells, two to four cells and four to eight cells were especially telling. For instance, a two-cell embryo that started to divide but then rested at the three-cell stage for several hours had less potential than an embryo in which both cells divided more synchronously (within an hour) forming a four-cell embryo. Time-lapse imaging allowed us to precisely monitor cell cycle length, time for each blastomere to complete its division, and the resting phase of the embryo.
Advertisement
Also interesting were some of the cleavage anomalies that we witnessed for the first time. These included division of a single cell in to three blastomeres, reverse cleavage where a blastomere was reabsorbed, and the presence of multiple nuclei within a single cell. Deselection of embryos with these traits is now routinely performed in our laboratory.
From our initial work we concluded that early morphokinetic parameters differ between embryos that make good-quality blastocysts and those that fail to blastulate or that show poor blastocyst morphology. Even among good-quality transferred blastocysts, we were able to detect differences between implanting versus nonimplanting blastocysts using time-lapse data. This latter observation may be especially valuable as our laboratory moves toward elective single-embryo transfer.
The EmbryoScope and future generations of time-lapse systems will revolutionize the laboratory practice of embryology. Such systems allow hands-free monitoring of embryo growth and development of computer algorithms for enhanced embryo selection. The remote access capability of such systems means information can be easily shared with the fertility team.
For physicians, time-lapse videos may prove an invaluable tool in counseling patients and helping them understand IVF failures. This leading-edge technology has already become integral to IVF at Cleveland Clinic, leading to increasing pregnancy and implantation rates.
Reference
1. Desai N, Ploskonka S, Goodman LR, Austin C, Goldberg J, Falcone T. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. 2014;12:54.
Advertisement
Photo © Russell Lee Photography
Advertisement

Uterine transposition cleared the field for radiation therapy

ACOG-informed guidance considers mothers and babies

Prolapse surgery need not automatically mean hysterectomy

Artesunate ointment shows promise as a non-surgical alternative

New guidelines update recommendations

Two blood tests improve risk in assessment after ovarian ultrasound

Recent research underscores association between BV and sexual activity

Psychological care can be a crucial component of medical treatment