Epidemiological studies lay the groundwork for future clinical investigations
By Hany Aly, MD; Allison Peluso, MD; and Raed Bou Matar, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Investigators at Cleveland Clinic Children’s conducted several epidemiological studies utilizing the Healthcare Cost and Utilization Project (HCUP) dataset, sponsored by the Agency for Healthcare Research and Quality.
The National Inpatient Sample, produced as part of the HCUP, contains over 7 million admissions each year. This large dataset enables the evaluation of epidemiological factors and population trends on a national scale to potentially identify risk factors and outcomes.
The robust data allow multiyear trend analysis, evaluation of outcomes among different races/ethnicities, as well as healthcare cost and utilization for conditions and interventions that range from rare to moderately common.
Prematurity and congenital heart disease (CHD), independently or concurrently, cause the majority of mortality and morbidity incidence among neonates in the United States. Prematurity is defined as birth before 37 weeks of gestational age. The March of Dimes reports the rate of prematurity for the United States as 10.6% in 2020 with a range for individual states of 8.2-%-14.6%.1 CHD is the most common congenital anomaly with a wide range of severity. The incidence of moderate and severe forms of CHD is 6/1,000 live births.2
The first study we conducted was on the prevalence and outcomes of acute kidney injury (AKI).3 Using the database, we identified 19,603 infants with AKI. The majority (74.3%) of the infants with AKI weighed < 1000g and 63.9% were ≤ 28 weeks of gestation.
The prevalence of AKI varied according to ethnicity. The occurrence was significantly lower in White populations than in Black and Hispanic populations (P < 0.001) and, further, the prevalence in Asian and Pacific Islander populations was considerably lower than in White populations (P < 0.001).
Advertisement
AKI was more frequently observed in male infants. Over the years, AKI incidence significantly increased in the overall population (Z score= 4.33, P < 0.001) and in the two birth weight categories: < 1,000g (Z= 11.75, P < 0.001) and 1,000 to 1,499g (Z= 3.18, P <0.001)] (Figure 1).
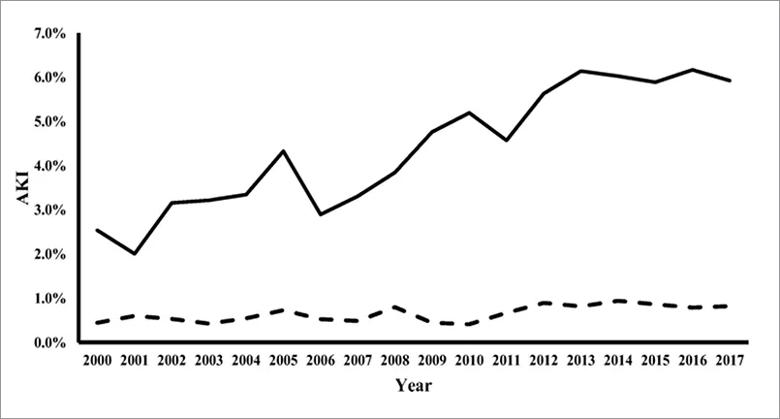
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ff405fc9-af53-4a5a-8283-a8bccd7f0edf/Inset-1_jpg)
Figure 1. AKI trend 2000- 2017. The solid line denotes birth weight < 1000 g (Z score = 11.75, p < 0.001) and the dotted line denotes birth weight 1000 to 1499 g (Z score = 3.18, p = 0.001). The trend of AKI frequency increased in both categories.3 Reprinted by permission from Springer Nature: Elgendy, M.M., Othman, H.F., Younis, M. et al. Pediatr Nephrol 36, 2789–2795 (2021).
Infants diagnosed with AKI who were preterm and with a lower birth weight had significantly higher mortality, longer hospital stay (58 vs. 35 days, P < 0.001) and a higher cost of hospitalization (over $360,000) (P < 0.001). A population analysis further revealed that comorbidities, such as intraventricular hemorrhage, (OR= 5.16, CI: 4.97-5.36), retinopathy of prematurity (OR= 1.36, CI: 1.32-1.42) and bronchopulmonary dysplasia (OR = 2.97, CI 2.88-3.06), were higher in the AKI group. The infants in the AKI group also had a significant increase in the prevalence of patent ductus arteriosus (OR = 3.97, CI: 3.69-3.90 P < 0.001).
We also used the database to report the prevalence, morbidity and mortality among preterm infants receiving platelet transfusion.4 A total of 22,609 infants were diagnosed with thrombocytopenia and 5,134 of them received platelet transfusion. Among the ones who received a transfusion, 60.3% were born before 28 weeks of gestation.
Advertisement
Platelet transfusion was associated with increased mortality in infants < 1,000g (aOR= 1.95, CI: 1.59- 2.49, P < 0.001) and in infants with birth weight 1,000 -1,499g (aOR = 1.99, CI: 1.59- 2.49, P < 0.001). The transfusion group had a longer duration of hospital stay (51 vs. 47 days) and increased average healthcare and hospitalization costs ($298,204 vs. $219,760). We also noted a significantly higher frequency of other comorbidities, like necrotizing enterocolitis (OR=1.67, CI: 1.51-1.86, P < 0.001), retinopathy of prematurity (OR = 1.16, CI: 1.07-1.25, P < 0.001) and AKI (OR=1.94, CI: 1.71-2.20, P < 0.001), compared to the group that did not receive platelet transfusion (Table 1).
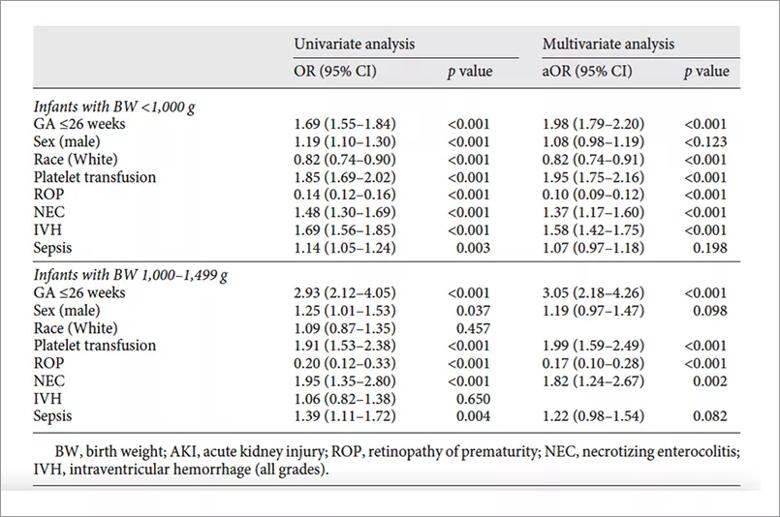
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d01a0fa5-8cf1-432b-97b8-d043ef7053fd/Inset-2_jpg)
Table 1. Regression analysis for factors associated with mortality for platelet transfusion in preterm infants.4 Copyright © 2021 Karger Publishers, Basel, Switzerland. Elgendy MM, Durgham R, Othman HF, Heis F, Abu-Shaweesh G, Saker F, Karnati S, Aly H. Platelet Transfusion and Outcomes of Preterm Infants: A Cross-Sectional Study. Neonatology. 2021;11:1-9.
A large dataset, like the NIS, is especially useful when studying rare diseases, such as Ebstein’s anomaly (EA). This rare CHD accounts for approximately 1% of all CHD cases. We analyzed the database from 2000-2018 and identified 4,398 infants with EA or EA with pulmonary stenosis or pulmonary atresia.5
The prevalence was 7/100,000 admissions (0.0064%). Isolated EA had fewer chromosomal anomalies detected (OR < 1, P < 0.001), and mortality for neonates with EA (12.3%) was considerably higher than non-EA critical congenital heart defects (CCHD) (OR= 1.45, CI: 1.33-1.59, P < 0.001).
Advertisement
Surgical repair was performed on 4.2% of neonates with EA. The majority of deaths (86.6%, N = 470) were observed in neonates devoid of corrective surgeries. The average length of hospital stay was seven days for this group, which was shorter than non-EA CCHD. Evaluation revealed that comorbidities in this cohort include conduction disorders (OR = 2.39, CI: 2.07-2.75, P < 0.001), arrhythmias (OR = 1.86, CI: 1.69-2.05, P < 0.001), respiratory failure (OR=0.66, CI: 0.57-0.77, P < 0.001), acute renal failure (OR = 1.42, CI: 1.25-1.60, P < 0.001) and intraventricular hemorrhage (OR = 0.52, CI: 0.42-0.63, P < 0.001).
We are hopeful that this epidemiological research lays the groundwork for further clinical research that examines risk reduction strategies of adverse outcomes in the most vulnerable pediatric populations. Furthermore, a better understanding of population outcomes allows for future interventions designed to reduce differences in healthcare delivery and outcomes.
References
1. March of Dimes. 2020 March of Dimes Report Card. https://www.marchofdimes.org/mission/reportcard.aspx
2. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Card. 2002;39:1890-1900.
3. Elgendy MM, Othman HF, Younis M, Puthuraya S, Matar RB, Aly H. Trends and racial disparities for acute kidney injury in premature infants: the US national database. Pediatr Nephrol. 2021;36(9):2789-2795.
4. Elgendy MM, Durgham R, Othman HF, Heis F, Abu-Shaweesh G, Saker F, Karnati S, Aly H. Platelet Transfusion and Outcomes of Preterm Infants: A Cross-Sectional Study. Neonatology. 2021;11:1-9.
Advertisement
5. Peluso AM, Othman HF, Zahka K, Perez AL, Sammour I, Aly H. Neonatal Ebstein anomaly national outcomes from 2000 to 2018 using the National Inpatient Sample. Birth Defects Res. 2021;113(14):1037-1043.
About the authors: Dr. Aly is Chair of the Department of Neonatology at Cleveland Clinic Children’s and Professor of Pediatrics, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
Dr. Peluso is a neonatologist in the Department of Neonatology at Cleveland Clinic Children’s and Assistant Professor of Pediatrics, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
Dr. Bou Matar is Medical Director, Pediatric Dialysis in the Center for Pediatric Nephrology and Hypertension at Cleveland Clinic Children’s and Assistant Professor of Pediatrics, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
Advertisement

One pediatric urologist’s quest to improve the status quo

Overcoming barriers to implementing clinical trials

Interim results of RUBY study also indicate improved physical function and quality of life

Innovative hardware and AI algorithms aim to detect cardiovascular decline sooner

The benefits of this emerging surgical technology

Integrated care model reduces length of stay, improves outpatient pain management

A closer look at the impact on procedures and patient outcomes

Experts advise thorough assessment of right ventricle and reinforcement of tricuspid valve