Novel exhalation delivery system with fluticasone improves outcomes for patients suffering from chronic rhinosinusitis with nasal polyps
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A recent article, published in the American Journal of Rhinology & Allergy, reports on a large multi-center randomized, placebo-controlled clinical trial of an exhalation delivery system with fluticasone (EDS-FLU) used to treat chronic rhinosinusitis (CRS) with nasal polyps. Compared to the placebo, EDS-FLU significantly improved nasal congestion/obstruction, polyp grade and a wide range of outcome measures, including quality of life, while eliminating nasal polyps in some patients.
Estimated to effect 12.5 percent of the U.S. population, CRS with or without polyps is a common inflammatory disease that results in lost work days and frequent antibiotic prescriptions. Symptoms include facial pain and/or pressure, congestion and/or obstruction, rhinorrhea, and diminished or absent sense of smell, depression, sleep impairment, head and body aches, and fatigue.
The current clinical treatment of CRS with nasal polyps, intranasal steroids, often provides suboptimal outcomes. Even surgery does not cure the problem in the majority of patients with polyps. As a result, substantial opportunity exists for improved medical care of chronic rhinosinusitis.
In CRS, chronically inflamed epithelial surfaces, frequently above the inferior turbinate bone and behind the nasal valve and uncinate process, impair ventilation and drainage through sinus osita. There may be nasal polyps (i.e., benign lesions arising from chronically inflamed tissue) in the middle meatus or ostiomeatal complex.
Reaching this area with topical steroids via current nasal sprays can be difficult, leaving the inflamed and obstructed areas undertreated. Surgery has proven cost effective in that it improves quality of life scores, but does not always fully or permanently resolve symptoms. Moreover, in many patients the polyps grow back over time. Opportunity exists to develop a reliable means to place high-potency topical steroid in target regions on a long-term outpatient basis.
Advertisement
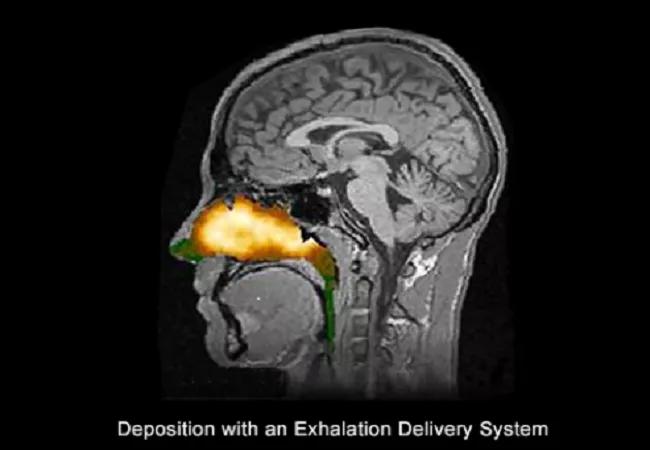
The novel exhalation delivery system exploits a balanced closure of the soft palate, delivering medication broadly behind the nasal valve and head of the inferior turbinate. The fluticasone formulation is approximately twice the concentration available in Flonase®, and has no alcohol or fragrance.
We were part of a multi-center clinical trial, in which a 16-week double-blind, randomized clinical trial with fluticasone (EDS-FLU) was conducted to determine the efficacy of increasing superior/posterior intranasal corticosteroid deposition. Study participants (n=323) were over 18 years of age, with moderate or greater nasal congestion/obstruction as reported by patient for at least five of the seven days leading up to screening, and a nasal polyp grade of one to three in each nasal cavity. Approximately 95 percent of participants had previously used steroids for their nasal issues, and notably, 60.4 percent had undergone previous sinus surgery, including polypectomy.
Nearly 91 percent of participants completed the double-blind treatment phase, with 282 of them entering an eight-week extension during which all participants received EDS-FLU twice daily. Participants received EDS-placebo, EDS-FLU 96 µg, EDS-FLU 186 µg or EDS-FLU 372 µg. Safety assessments (i.e., nasal endoscopy) were conducted at four-week intervals at the initial screening, and during the 16-week period. Ophthalmologist screening was conducted at weeks 16 and 24 for intraocular pressure (OPE) and cataract presence.
Primary outcomes included objective nasal polyp grade as well as morning congestion score and symptoms as measured by a validated QOL survey. All three doses of EDS-FLU improved morning congestion score and mean change in endoscopically assessed total polyp grade compared to participants receiving EDS-placebo.
Advertisement
Relief of morning congestion increased with the dose of EDS-FLU, with the highest dose resulted in largest mean reduction. However, differences between the doses were not statistically insignificant. Results from other subjective outcomes measures, including the validated Sino-Nasal Outcome Test-22 (SNOT-22) and Sleep-R disturbance scores, substantial improvement in EDS-FLU groups. SNOT-22 and Sleep-R scores also improved, though not as much, in the FLU-placebo group, indicating that the delivery mechanism itself may have a positive effect on patient symptoms.
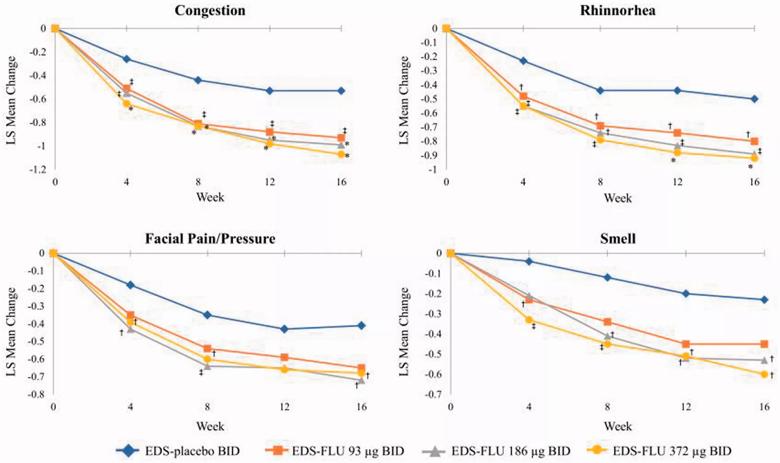
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6af92f99-36b3-4c0c-aec8-f2074c2f23a0/figure-5_jpeg)
Mean change in AM instantaneous symptoms of congestion, facial pain and pressure, rhinorrhea, and sense of smell at weeks 4, 8, 12, and 16. AM, morning; BID, twice daily; EDS-FLU, exhalation delivery system with fluticasone; LS, least squares. †P 0.05 versus EDS-placebo. ‡P 0.01 versus EDS-placebo. *P 0.001 versus EDS-placebo.
Adverse events possibly attributable to EDS-FLU were generally local: epistaxis, nasal mucosal disorder, acute sinusitis, upper respiratory tract infection, nasal congestion, nasal septum, ulceration, nasopharyngitis and gastrointestinal disorders. Two cases of nasal septum perforations were identified on endoscopy in EDS-FLU patients (0.8 percent of all EDS-FLU patients). Both had undergone previous sinonasal surgery, which is an independent risk factor for perforation.
EDU-FLU uses a new mechanism that deposits medication high and deep in the nasal cavity directly at the sites of inflammation, producing meaningful improvement in a broad range of objective and subjective outcome measures in patients with nasal polyps.
Advertisement
Note: Images used under Creative Commons license; originally published here: Sindwani R, Han J, Soteres D, Messina J, Carothers J, Mahmoud R, Djupesland P. NAVIGATE 1: Randomized, placebo-controlled, double-blind trial of the exhalation delivery system with fluticasone for chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2019 Jan;33(1):69-82.
Advertisement
Advertisement

Analysis of HNSCC patients shows HPV status to be predictive of higher abundance of oncobacteria within the tumor

Case study illustrates the potential of a dual-subspecialist approach

Evidence-based recommendations for balancing cancer control with quality of life

Study shows no negative impact for individuals with better contralateral ear performance

HNS device offers new solution for those struggling with CPAP

Patient with cerebral palsy undergoes life-saving tumor resection

Specialists are increasingly relying on otolaryngologists for evaluation and treatment of the complex condition

Detailed surgical process uncovers extensive middle ear damage causing severe pain and pressure.