Early communication between oncologists and ophthalmologist warranted
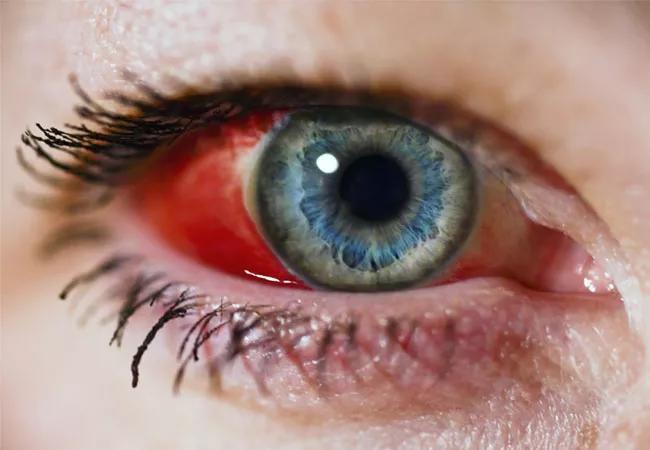
Newer treatments such as monoclonal antibodies, immunotherapies, antibody-drug conjugates, growth factor receptors, check point inhibitors and targeted therapies improve the prognosis for many types of cancer. Yet in the real-world setting, cases of ocular side effects have emerged in greater frequency both in new and established therapies than were seen in many clinical trials.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Researchers at Cleveland Clinic’s Taussig Cancer Center have recently published manuscripts that compile ocular toxicities of several anti-cancer drugs in the hopes of being able to predict who will be impacted by these adverse events and what treatments and interventions can mitigate these risks. The researchers analyzed patient cases as well as prior medical literature to better understand ocular complications that have occurred in patients taking antineoplastic agents.
“As clinicians, we need to look at not just the efficacy of a treatment but what toxicities are occurring to vital organs like the eyes and the impact this has on that patient’s quality of life,” says Shahzad Raza, MD, a physician at Taussig Cancer Center’s Department of Hematology and Medical Oncology and senior author of the study. “Patients struggle because they don’t know how to navigate the situation, and physicians are often unsure what steps to take to alleviate ocular toxicities. Ophthalmologists should be involved much earlier in treatment decision making and the potential risks to the eyes.”
Currently, there are many unknowns about how to manage these issues, such as when to pause treatment and when to reintroduce it. The research team combined in-depth clinical data about how these drug toxicities occur with published literature to develop a reference tool for providers in deciding what steps to take, such as holding medication, prescribing eye drops or other measures, when eye problems occur.
Among the findings in the study include the following:
Advertisement
Although eye toxicities are not the norm, they occur with more frequency than typically found in clinical trials, and in some cases they can occur rapidly. Depending on the therapy, temporary cessation of treatment or lowering of dosage can help reverse more severe toxicities but not always. Based on these findings, the researchers recommend if a patient is on a therapy where there is a risk of ocular side effects that their oncologist:
“In some cases, eye issues occur gradually but in some instances such as retinal detachment, it can happen right away and become an emergency,” says Dr. Raza. “If a patient is just starting to reach out to an ophthalmologist when these toxicities occur, often the damage has already been done and by the time their oncologist is holding the drug, the cancer progresses. The moment an issue emerges, there should be a lifeline to an ophthalmologist.”
Dr. Raza and a team of computational mathematicians are using data mining to evaluate oncology drug toxicity trends overall. By mining national patient databases of thousands of patients, they hope to discover how factors such as race, ethnicity, drug dosing and gene modification play in drug toxicities. “What we don’t yet understand is why one patient has a side effect and another does not,” he explains. “What factors are involved? Some may be inherent genetics but we feel there are particular pharmacokinetics and pharmacogenomics signals involved that may amplify certain toxicities.”
Advertisement
Ultimately, the goal is to bring pharmacovigilance and genomics together to better predict when toxicities will occur so that clinicians can tailor treatment accordingly.
Advertisement
Advertisement

Key themes and insights into the family-caregiver experience

First-of-its-kind clinic for immune-related adverse events supports oncologists in managing severe side effects

Variables affect nuances of the conversation

Driving advances in cancer care
Challenges emerge with application of interim PET scans

Complex, bidirectional interactions exist between cancer, therapy, toxicities and eating behaviors

Helping patients with cancer struggling with depression, anxiety and other mental health issues

How ‘Let’s Get Moving!’ is improving physical activity in children undergoing cancer treatment