Surgeons completed radical prostatectomy using new-generation robot
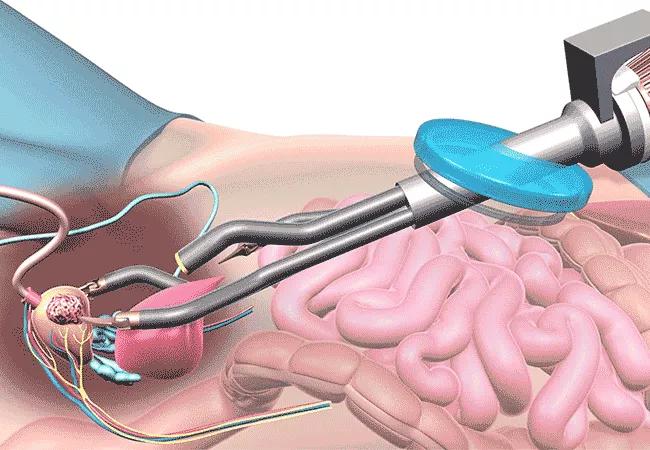
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3902241c-11dd-4272-a885-a1f299633bca/SP-Port-Robot-1-650x450_gif)
SP-Port-Robot-1-650×450
Cleveland Clinic is the first hospital in the country to successfully perform surgeries using the Single Port SP Robot, which inserts all surgical instruments through one small abdominal incision, improving surgical outcomes and allowing quicker patient recovery.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
On Sept. 28, Cleveland Clinic surgeons used the SP Robot to perform three surgeries — two surgeries to remove cancerous prostates and one surgery to remove an enlarged prostate blocking the urinary system through the bladder.
Jihad Kaouk, MD, Director of the Center for Robotic and Image Guided Surgery in the Glickman Urologic and Kidney Institute, was the first to perform and publish on robotic single-port surgery in 2008 using standard robotic systems and coining the phrase R-LESS (robotic laparoendoscopic single site surgery).
After completing and publishing the first ever clinical use for the SP robot in Europe, Dr. Kaouk and his team also performed last week’s radical prostatectomies and transvesical simple prostatectomy at Cleveland Clinic. The new purpose-built robotic SP system will allow the single port approach to be more feasible.
“We anticipate that this new generation of robots will allow for new and different routes of surgeries that haven’t previously been possible,” Dr. Kaouk says. “For example, we can now go through a patient’s perineum instead of their belly to perform prostate surgery and avoid touching the bowel, or work through the retroperitoneal space to perform kidney surgery without entering the abdomen, allowing for quicker recovery time.”
Currently, the SP Robot is only FDA-approved for urologic surgeries, with plans to expand to ENT and colorectal surgeries in the near future.
“We are proud to offer this surgical approach and be on the forefront of surgical innovation,” adds Mark A. Taylor, MD, Chairman of Surgical Operations at Cleveland Clinic.
Advertisement
Dr. Kaouk worked with the Intuitive Inc. team of engineers to test and improve the new robotic system. Dr. Kaouk is a paid consultant, speaker or member of the advisory committee for Endocare, Inc. and Intuitive Surgical, Inc.
Advertisement
Advertisement
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches