New protocol may lead to improved patient outcomes
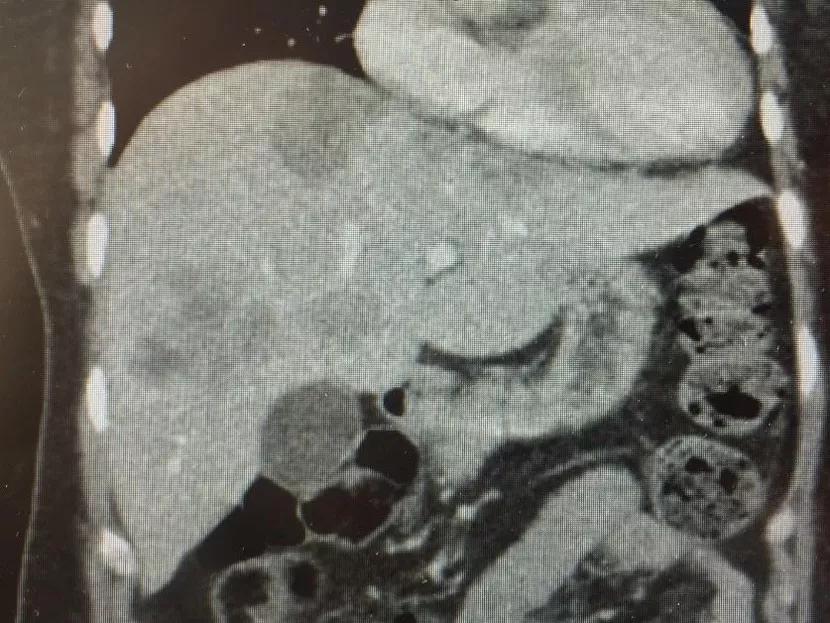
The prognosis for patients with liver metastases from colorectal cancer is bleak. Consider the following U.S. stats:
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Most treatment options for liver metastases from colorectal cancer don’t offer patients much hope. While the standard of care treatment for this condition is liver resection, only a third of these patients are candidates for surgery. (Surgery provides a five-year survival rate of 25 to 60 percent for this small subset of patients.) The remaining majority are treated with systemic chemotherapy as the standard of care. However, the five-year survival rate for systemic chemotherapy is only about 10 percent, with a median survival of about 24 months.
To improve patient outcomes, physician-researchers at Cleveland Clinic developed a new transplant protocol for treating liver metastases from colorectal cancer. Under the leadership of Federico Aucejo, MD, Surgical Director of Cleveland Clinic’s Liver Cancer Program, Cleveland Clinic surgeons implemented the protocol to treat a patient with unresectable liver metastases from colorectal cancer, marking the first time that liver transplant surgery was performed for this indication in the U.S.
“Incorporating liver transplantation as a treatment option would allow the surgical management of liver metastases that cannot be treated with liver resection,” explains Dr. Aucejo. “If we can demonstrate the survival benefit of this approach, we may be able to prolong the life of a significant number of patients.”
Cleveland Clinic began developing its new surgical protocol based on a 2011 pilot study of 21 patients at the University of Oslo in Norway. The results of the study showed that liver transplantation helped colorectal cancer patients with unresectable liver metastases achieve a five-year survival rate of 60 percent.
Advertisement
Once the new protocol was developed, Cleveland Clinic found a perfect candidate for the first surgical application. This patient had unresectable metastases in the liver as well as liver disease from long-term chemo toxicity. The patient’s cousin served as the live liver donor. Following the successful procedure, both patient and donor were discharged from the hospital within the expected time frame.
Through the Liver Cancer Program’s research efforts, Cleveland Clinic is studying liquid biopsy technology, a tool to help with the selection of patients with less aggressive tumor biology. “As we explore and refine this technique of analyzing circulating tumor DNA, we hope to overcome the inefficiency of current tumor biomarkers, allowing the identification of a subset of patients that may benefit the most from these advanced surgical protocols,” notes Dr. Aucejo.
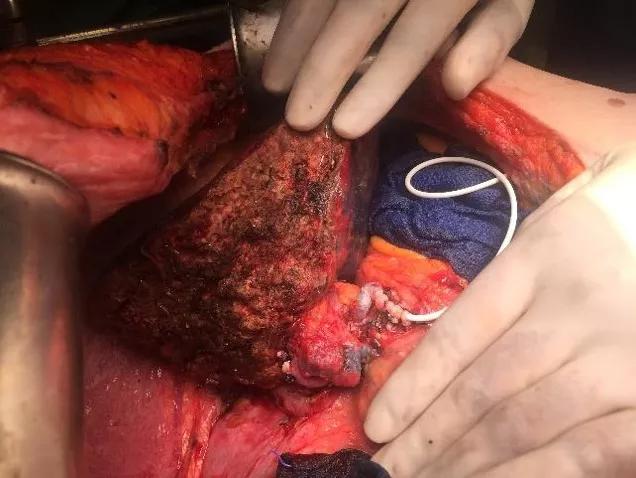
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7087f7c1-b24b-4443-859a-60829818d809/LiverTransProt-Fig-2_jpg)
Along with the new transplant protocol, the Liver Cancer Program incorporated a hepatic artery chemotherapy infusion pump protocol for patients with disease limited to the liver. So far, doctors have performed 13 pump implantations in combination with liver surgery to treat metastases from colorectal cancer. In addition to systemic chemotherapy, patients receive a chemotherapy pump that is implanted into the abdominal wall. The pump is connected to a catheter that is inserted into one of the arteries that connects to the liver.
By infusing chemotherapy directly into the liver, the infusion pump treats liver metastases more efficiently by preventing tumor recurrence after surgery or by reducing the bulk of disease so that it can be subsequently removed using surgery.
Advertisement
“Moving forward, we expect that both liver transplant and hepatic artery chemotherapy pump protocols will complement each other,” explains Dr. Aucejo. “Directed chemotherapy to the liver via an implantable pump will help limit disease to the liver over time. When chronic liver disease from long-term chemo toxicity develops, salvage liver transplantation will be the next step to consider.”
Now that the new protocol has been successfully used on a patient, the next phases involve proving its long-term efficacy and shifting to cadaver donors. “Initially, we are using live donors for transplants due to the uncertainty of the long-term oncological outcome. This uncertainty stems from the close association between immunosuppression therapy and post-transplant tumor recurrence,” explains Dr. Aucejo. “If our initial experience performing liver transplantation in patients with liver metastases from colorectal cancer using live donors is promising, accessing the cadaveric pool will become easier in the future.”
“From an oncological standpoint, our goal is to achieve a five-year survival rate of at least 50 percent,” reveals Dr. Aucejo. “If we can prove that the new protocol results in that kind of rate, we will be able to justify this indication for transplantation.”
“The whole concept of using advanced surgical protocols (e.g., liver resection complemented by both systemic and hepatic artery pump chemotherapy and liver transplantation) to treat liver metastases from colorectal cancer is a way to transform the condition from a fast-moving and fatal disease to a chronic condition with prolonged patient survival,” says Dr. Aucejo. “As a result, we speculate that by extending patient survival through aggressive surgical and locoregional therapies, novel and more effective systemic therapies will develop and increase survival even further at that time.”
Advertisement
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors