Knocking out, then restoring, the immune system holds great promise
The first North American clinical trial directly comparing stem cell transplantation with the most potent biologic therapies for treatment-resistant relapsing multiple sclerosis (MS) is underway. The phase 3 BEAT-MS investigation (NCT04047628), launched in December 2019, is assessing high-dose immunosuppression plus autologous hematopoietic stem cell transplantation (I/AHSCT) against any of several disease-modifying therapies (DMTs) approved for relapsing MS.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Despite the proliferation of effective drugs for MS in recent years, some patients with highly active disease do not respond well to available therapies and face a substantial risk of disability,” says the multicenter study’s national principal investigator, Jeffrey Cohen, MD, Director of the Experimental Therapeutics Program in Cleveland Clinic’s Mellen Center for MS Treatment and Research. “Immunosuppression followed by hematopoietic stem cell transplant has demonstrated potent and long-lasting efficacy in early trials and offers great promise for such patients.”
The six-year randomized controlled BEAT-MS trial is funded by the National Institute of Allergy and Infectious Diseases and conducted through the Immune Tolerance Network.
Many of the most potent DMTs currently used for relapsing MS are monoclonal antibodies that modulate or suppress autoimmune processes underlying the disease. In addition to having incomplete efficacy for some patients, DMTs do not reverse damage. These expensive medications also must be taken continuously and involve potential safety concerns whose risk accumulates over time.
Stem cell therapy is a rapidly advancing field aimed at addressing MS treatment gaps. For patients who already have nerve damage, therapies based on mesenchymal stem cells are being evaluated for promoting tissue repair and reversing disability. However, they do not stop MS-related inflammatory lesion activity.
In contrast to mesenchymal stem cell therapies, I/AHSCT aims to halt the inflammatory process, similar to the tack taken by DMTs. I/AHSCT is a complex therapy consisting of the following stages, as depicted in the figure below:
Advertisement
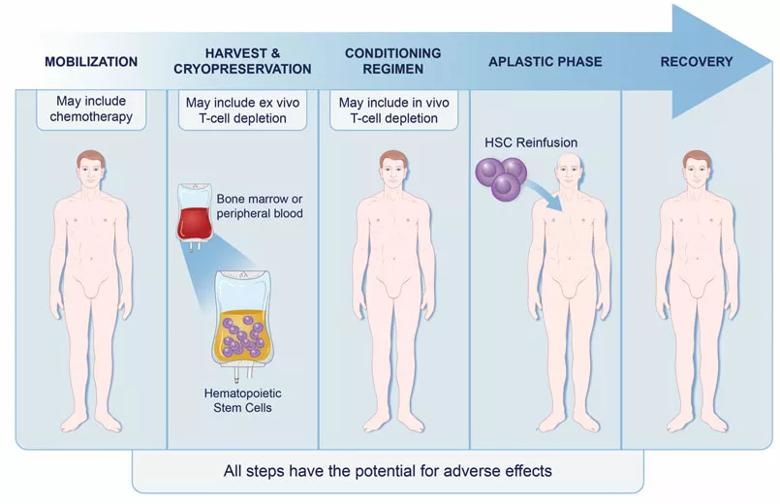
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/019a89ed-f180-48de-9a9a-afe6cab78e6a/19-NEU-6534-Cohen-stem-cells-for-MS_inset-805x520_jpg)
Figure. The stages of immunoablation followed by autologous hematopoietic stem cell transplantation for treatment of MS.
“The conditioning phase is the actual therapy for MS, as it provides potent immune suppression,” Dr. Cohen explains. “The subsequent stem cell transplantation reboots the patient’s immune system, but now in a milieu that no longer promotes MS activity.”
BEAT-MS will follow 156 patients for six years at 19 sites in the U.S. and the United Kingdom. Patients will be randomized to receive I/AHSCT or best available therapy, consisting of a DMT (natalizumab, alemtuzumab, ocrelizumab or rituximab) selected by the site investigator.
Patients must be 18 to 55 years old with highly active, treatment-resistant relapsing MS. The primary outcome measure is time of MS relapse-free survival up to three years; secondary measures include evidence of MS activity, disability worsening and improvement, changes in whole brain volume and serum neurofilament light chain concentrations, and adverse events. Exploratory outcomes will include advanced MRI measures, patient-reported quality of life, health economic analysis and immune mechanistic studies.
I/AHSCT has done well in previous clinical trials and retrospective studies, Dr. Cohen notes. Rates of NEDA (no evidence of disease activity) reached 70% to 90%, versus 50% for other MS therapies, and some patients remained stable for five to 10 years. Many patients demonstrated improved disability and MRI measures.
The results led Dr. Cohen to serve as lead author of a position statement from the American Society for Blood and Marrow Transplantation (Biol Blood Marrow Transplant. 2019;25:845-854) advocating that treatment-refractory relapsing MS with high risk of future disability be considered an indication for I/AHSCT.
Advertisement
He cautions, however, that available evidence supporting I/AHSCT includes various patient populations, therapeutic protocols and outcome measures, with some trials using no or suboptimal controls. He adds that four I/AHSCT trials similar to BEAT-MS are currently being conducted; BEAT-MS is the only one based in North America.
“A big unanswered question is how aggressive the conditioning regimen should be,” Dr. Cohen observes, noting that treatment-related mortality has occurred, but rarely. “A too-aggressive protocol compromises safety, while efficacy is diminished if it is not aggressive enough.”
BEAT-MS is taking an intermediate approach designed to optimally balance safety and efficacy, he explains. He adds that refinements to protocols and better patient selection have led to improved safety compared with early experience.
Dr. Cohen notes that the patients most likely to benefit from I/AHSCT are young and still ambulatory, have relatively recent disease onset, have highly active MS with recent clinical relapses and MRI lesion activity, and continue to have disease activity despite use of first- and second-line agents.
Although the cost of the therapy is high, patients who achieve long-term disease stability can avoid the continuous expense of DMTs, which could ultimately be cost-effective.
“Depending on the outcome of BEAT-MS and the other trials, we will consider advocating to expand the patient population recommended for this therapy,” Dr. Cohen concludes. “We may one day advise it earlier in a rapidly worsening disease course to maximize disability prevention.”
Advertisement
Advertisement

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists