Initial findings demonstrate improved symptoms and reduced steroid dependence
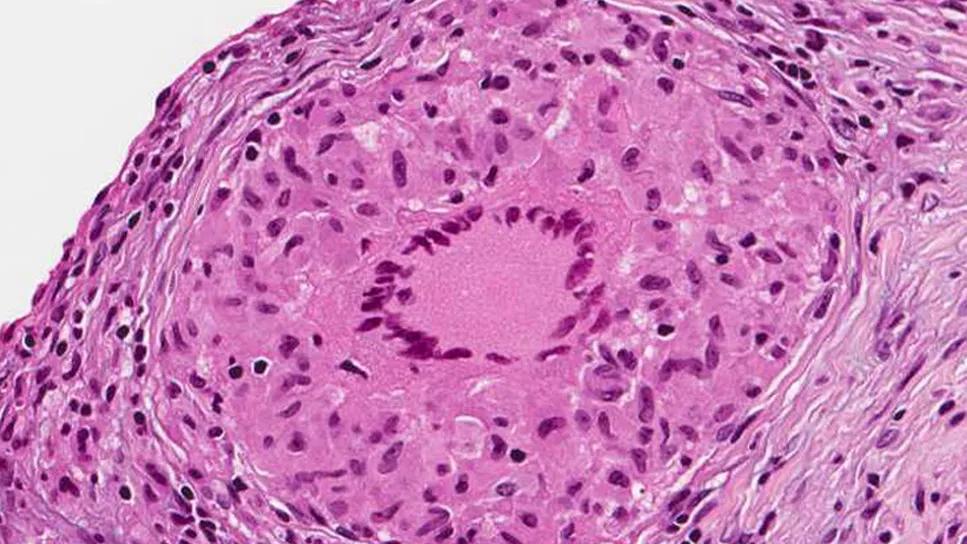
The current consensus standard of care for patients with sarcoidosis focuses primarily on oral glucocorticoids to help suppress inflammation. However, long-term glucocorticoid use is associated with several significant side effects including significant weight gain, insulin resistance and impaired quality of life. A recent presentation from the CHEST 2024 Meeting Insights dives deeper into a study originally appearing in Chest that explores the tolerability and effectiveness of efzofitimod (ATYR1923), a novel immunomodulator, as an alternative sarcoidosis treatment.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“Increasingly, there's been more of a focus on the role of innate immunity and of the monocyte-macrophage cell line in the development and progression of sarcoidosis,” says Daniel Culver, DO, FCCP, Chair of the Division of Pulmonary Medicine at Cleveland Clinic and the lead author of the original study. “The predominant cell in a granuloma is the epithelioid histiocyte, which is derived from monocyte-macrophage cells. There's an increasing understanding that macrophage monocyte plasticity and the phenotype of the inflammatory response in these cells may drive both the development and outcomes of granulomatous inflammation.”
He continues, “We all remember from our first biology classes that tRNA synthases are important for assembling proteins. However, it appears that through evolution, our tRNA synthases get longer and longer, which leads to secreted splice variants. Some of these secreted variants have extracellular functions, so they get released locally, which causes signaling functions, including signals modulating immune homeostasis.”
Dr. Culver explains that in these instances, the tRNA synthases are no longer involved with catalyzing amino acid addition, and they influence other signaling pathways instead. This transition is believed to be an important homeostatic mechanism that regulates immune responses, and efzofitimod takes advantage of this.
“Efzofitimod is a synthetic fragment of one of the tRNA synthase variants, which is conjugated to an Fc fragment,” says Dr. Culver. “It binds to a receptor, neuropilin 2, which normally functions as a heterodimer to regulate immune responses in a variety of cells.”
Advertisement
The original randomized, double-blind, placebo-controlled study published in CHEST focused on the tolerability, safety, and effect on outcomes of Efzofitimod in patients with pulmonary sarcoidosis. It evaluated multiple ascending doses of efzofitimod, administered intravenously every four weeks for 24 weeks. Randomized patients (2:1) underwent a steroid taper to 5 mg/d by week 8 or < 5 mg/d after week 16.
The study included 37 patients, and the authors found that the drug was well tolerated at all doses. The mean ± SD age of the patients was 52.4 ± 10.1 years, with 54% women, 62% white and 38% Black patients. Nine patients received placebo, and six, five, and eight patients received the 1 mg/kg, 3 mg/kg, and 5 mg/kg efzofitimod doses, respectively.
The average daily steroid dose through the end of the study were 6.8 mg, 6.5 mg, and 5.6 mg for the 1 mg/kg, 3 mg/kg, and 5 mg/kg groups, respectively. Alternatively, the placebo group received 7.2 mg for placebo, which resulted in a baseline-adjusted relative steroid reduction of 5%, 9%, and 22%, respectively.
The group did not observe any deaths or serious drug-related adverse events (AEs)in the study, and they found no connection between AE frequency and increased efzofitimod dosage. AEs within the respiratory system disorder were most common (cough, wheezing, dysphonia, upper airway infection), though they were mild in severity, and their occurrence seemed to be independent of the doing groups.
While AEs appeared to be unrelated to efzofitimod dosage, the observers noted that patient groups receiving the two highest dosages (3 mg/kg and 5 mg/kg) of the drug resulted in improvements in key lung function parameters at week 24 from baseline compared with placebo. The 5 mg/kg dose arm showed clinically meaningful and statistically significant improvements across several patient-reported outcomes. These included significant improvements in SAT lung (observed by week 12), KSQ lung (observed by week 8), KSQ GH (observed by week 4), and FAS (observed by week 8) compared with placebo.
Advertisement
“I think it is striking that there is a little bit better reduction of steroids as the dosing goes up,” says Dr. Culver. “In the highest dosage group, three patients (33%) were able to taper off steroids completely and maintain this reduction through the end of the study.”
The group also notes that while the improvements in FVC % predicted and DLCO % predicted did not achieve statistical significance, this may be due in part to the study’s limited sample size. However, Dr. Culver says, “The trends we observed signify the possibility of biological activity, and this is something that should be investigated further in a larger future study.”
Dr. Culver says that the group has finished enrolling patients in a larger Phase III study (EFZO-FIT), and he hopes to report those findings later this year. “This will be the largest prospective interventional study of sarcoidosis and the first-ever Phase III trial. We expect to learn a lot about how sarcoidosis in this kind of population behaves, regardless of the results of the trial.”
Advertisement
Advertisement

Nature of disease poses challenges for patients and clinicians

Recent breakthroughs have brought attention to a previously overlooked condition

A review of treatment options for patients who may not qualify for surgery

Looking at the real-world impact and the future pipeline of targeted therapies

The progressive training program aims to help clinicians improve patient care

New breakthroughs are shaping the future of COPD management and offering hope for challenging cases

Exploring the impact of chronic cough from daily life to innovative medical solutions

How Cleveland Clinic transformed a single ultrasound machine into a cutting-edge, hospital-wide POCUS program