The clinical picture
By Gursimran S. Kochhar, MD, Maged Rizk, MD, Deepa T. Patil, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
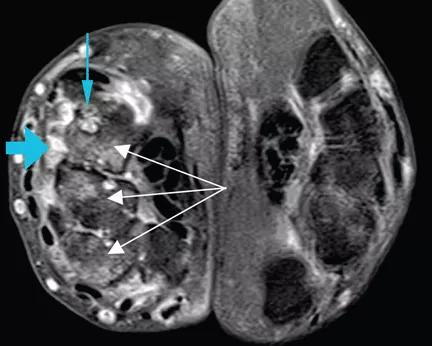
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions. At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (See image above: Axial T1-weighted magnetic resonance imaging with gadolinium contrast shows synovitis (large blue arrow) along the dorsal aspect of the wrist. Also seen are erosions in the carpal bones (thin blue arrow) and bone marrow edema (white arrows), which is asymmetrical compared with the other wrist, a finding highly suggestive of rheumatoid arthritis.). He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added. Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (Figure) as part of his workup for chronic abdominal pain.
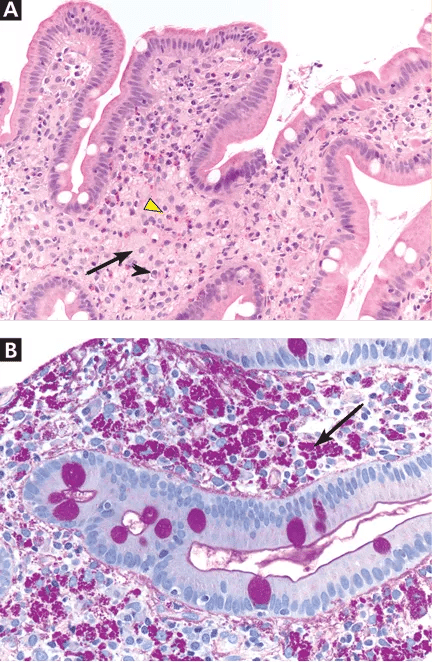
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2e184f1e-14bb-4ae7-80e0-36cde2879aca/RTEmagicC_Kochhar_WhippleDisease_F2_gif_gif)
Figure. (A) The duodenal mucosa shows expansion of the lamina propria by “foamy“ macrophages (black arrow) admixed with eosinophils (yellow arrowhead) and plasma cells (black arrowhead) (hematoxylin and eosin, × 100). (B) Periodic acid-Schiff staining with diastase digestion reveals foamy macrophages containing diastase-resistant bacilli (arrow) (× 200).
Advertisement
A: As shown in the figure above, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
Advertisement
This article originally appeared in the Cleveland Clinic Journal of Medicine, 2013 May;80(5):272-273.
References
Advertisement
Advertisement

Multidisciplinary framework ensures safe weight loss, prevents sarcopenia and enhances adherence

Study reveals key differences between antibiotics, but treatment decisions should still consider patient factors

Key points highlight the critical role of surveillance, as well as opportunities for further advancement in genetic counseling

Potentially cost-effective addition to standard GERD management in post-transplant patients

Findings could help clinicians make more informed decisions about medication recommendations

Insights from Dr. de Buck on his background, colorectal surgery and the future of IBD care

Retrospective analysis looks at data from more than 5000 patients across 40 years

Surgical intervention linked to increased lifespan and reduced complications