An 86-year-old man presented with unexplained right-sided headache and vision loss
By Marc Ohlhausen, BS; Eden Bernstein, MD; and Craig D. Nielsen, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This article is reprinted from the September 2021 issue of Cleveland Clinic Journal of Medicine (2021;88[9]:494-501).
An 86-year-old man presented to a local hospital ophthalmologist with headache and pain in his right temple without vision loss. Laboratory values for complete blood cell count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were normal. The medical history was remarkable only for a remote history of diverticulitis. He reported social consumption of alcohol and smoking in the past.
Although reassured, the patient returned two weeks later with acute loss of vision in his right eye, preceded by eye discomfort, floaters, flashing lights, and worsening right temporal pain and headache. On physical examination, he had no fever and was normotensive. Extraocular movements and visual fields were normal, and visual acuity was unchanged. Heart, lung, joint and skin examinations were unremarkable.
Given the patient’s age and presentation, giant cell arteritis (GCA) was suspected. Key clinical features of GCA in patients over age 50 include abrupt new headache, scalp pain and tenderness, jaw claudication, visual symptoms, polymyalgia rheumatica, temporal artery abnormalities, and elevated ESR or CRP, or both.1,2 Anterior ischemic optic neuropathy due to occlusion of the posterior ciliary artery is the cause in 85% of cases of vision loss in GCA.3
1. What is the most appropriate first step for a patient with suspected GCA?
Advertisement
If GCA is strongly suspected, high-dose systemic glucocorticoids should be started promptly to prevent irreversible vision loss and involvement of the other eye.4 Urgent referral for specialist management, ophthalmologic assessment and temporal artery biopsy are recommended but should not delay administration of glucocorticoids. Temporal artery biopsy is the preferred method of confirming GCA, although a negative result does not rule out disease in cases of high clinical suspicion.1
Initiating glucocorticoids should lead to significant improvement in symptoms. If this does not occur, one should evaluate for alternative diagnoses.
The patient was started on oral prednisone 60 mg for presumed GCA and admitted to the local hospital. ESR and CRP were repeated, and results again were within normal range. A right-sided temporal artery biopsy was performed but showed no evidence of active or healed arteritis. Magnetic resonance imaging (MRI) and computed tomography (CT) angiographic imaging of the head and neck were unremarkable.
The patient was discharged. The diagnosis of GCA was considered unlikely, but prednisone was continued out of concern for possible vision loss in the unaffected eye while other causes were being evaluated. Prednisone was up-titrated to 70 mg due to lack of symptom improvement, with no improvement in vision or headache severity reported.
The patient was referred to the ophthalmology department at a nearby large academic hospital for further workup. On presentation one month after his hospitalization, a variety of new signs and symptoms had developed. Ophthalmologic examination revealed right-sided ptosis, eyelid swelling and chemosis (i.e., swelling of the conjunctiva). The right eye was unable to gaze superiorly, inferiorly or laterally. Vision in the right eye was completely absent, with no light perception. The right pupil was nonreactive to light, and an afferent pupillary defect was present. The fundus appeared normal on dilated fundus examination. Intraocular pressures were 20 mm Hg (reference range 12–22 mm Hg) in both eyes. Tenderness to palpation was noted over the right eyebrow, temple and forehead. All findings in the left eye were normal.
Advertisement
The patient was admitted for additional workup. Initial laboratory investigations revealed a white blood cell count of 10.47 × 109/L (reference range 3.70–11.00 × 109/L) with 90.9% neutrophils, 5.9% lymphocytes, 3.1% monocytes, 0% eosinophils and 0.1% basophils. A complete metabolic panel was within normal limits except for an elevated blood glucose level of 170 mg/dL (reference range 74–99 mg/dL), which was attributed to steroid therapy. The ESR was 4 mm/hour (reference range < 30 mm/hour) and CRP was 0.15 mg/dL (reference range < 0.30 mg/dL).
Given the lack of response to prednisone, the normal ESR and CRP levels, and the negative temporal artery biopsy, the diagnosis of GCA was ruled out, and evaluation for other causes continued.
2. Involvement of the neural structures at which of these locations best explains the pattern of cranial nerve deficits seen on this patient’s examination?
The pattern of neurologic deficits localizing unilaterally to cranial nerves II (optic), III (oculomotor), IV (trochlear) and VI (abducens) seen in this patient is most consistent with disruption of structures at the orbital apex.5 The orbital apex is the posterior-most end of the orbit and is made up of bony, neural and vascular structures (Figure 1). Within the orbital apex are two orifices in the sphenoid bone:
Advertisement
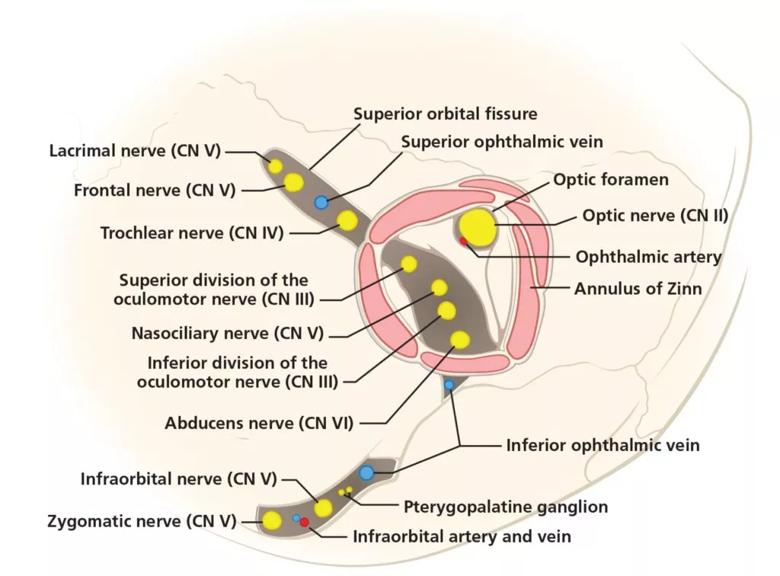
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/966dbcc4-30aa-4f4a-bb64-52189535d9f4/F1_large_-1024x756_jpg)
Figure 1. The anatomy of the orbital apex, the most posterior aspect of the orbit. The unilateral cranial nerve deficits in this patient point to disruption of structures of the orbital apex including the optic, oculomotor, trochlear and abducens nerves.
The annulus of Zinn is the common tendinous origin of the recti muscles and surrounds the optic foramen and the central portion of the superior orbital fissure. The contents of this annulus are the optic nerve, ophthalmic artery, oculomotor nerve, abducens nerve and nasociliary nerve. Because of their confinement, these structures are at greater risk of compression or shear injury.6
The presence of multiple nerve palsies in this patient’s presentation indicated that his condition was unlikely due to a primary pathology at the retina or optic nerve.7 In addition, on dilated fundus examination there was no optic nerve pallor and no finding suggestive of central retinal artery occlusion or retinal detachment.
3. Which of these syndromes is most likely to cause optic nerve dysfunction?
Orbital apex syndrome is the constellation of signs and symptoms resulting from a disease process affecting the orbital apex structures characterized by involvement of cranial nerves II, III, IV, VI and V1. The most common presenting features are vision loss, ophthalmoplegia and blurred vision.8,9 Involvement of the oculomotor, abducens and trochlear nerves causes ophthalmoplegia and diplopia owing to disruption of innervation to the extraocular muscles. Oculomotor nerve palsy also causes ipsilateral ptosis and mydriasis. Involvement of cranial nerve V1 results in hypoesthesia or pain of the ipsilateral forehead and upper eyelid, along with absence of corneal reflex and sensation. Inflammation due to infectious, inflammatory or neoplastic processes may cause proptosis. Variations in presentation of orbital apex syndrome are common owing to the large number of structures involved.
Advertisement
Due to close anatomic proximity, overlapping clinical features are found in two additional syndromes: cavernous sinus syndrome and superior orbital fissure syndrome (also known as Rochon-Duvigneaud syndrome).8-11 The names of these three syndromes correlate with the anatomic location of their disease processes.
Cavernous sinus syndrome presents similarly to orbital apex syndrome but also involves the maxillary nerve (V2). This results in hypoesthesia of the cheek and lower eyelid, along with facial pain extending farther inferiorly than the periorbital region innervated by the ophthalmic branch.10 On examination, the most useful distinction between cavernous sinus syndrome and orbital apex syndrome is involvement of the optic nerve, which is rare in cavernous sinus syndrome.11 Also of note, cavernous sinus syndrome may cause Horner syndrome (manifested by ptosis, miosis and anhidrosis) due to the involvement of the sympathetic chain adjacent to the cavernous segment of the internal carotid artery. Vascular etiologies of cavernous sinus syndrome are classically associated with a pulsatile proptosis.8
Superior orbital fissure syndrome occurs with a lesion directly anterior to the orbital apex, affecting structures coursing through the superior orbital fissure at this location. The presentation is similar to that of orbital apex syndrome but without optic nerve impairment. Superior orbital fissure syndrome can be progressive, and patients may go on to develop orbital apex syndrome or cavernous sinus syndrome.8
Possible causes of orbital apex syndrome are numerous and varied.8,10 In the absence of recent surgery or trauma, inflammatory, infectious, vascular and neoplastic etiologies must be considered.
Tolosa-Hunt syndrome is a common inflammatory cause of orbital apex syndrome. It presents with periorbital pain and limited eye movements, most often unilaterally.
Immunoglobulin G4-related disease is a systemic inflammatory condition that can include salivary gland enlargement, lymphadenopathy, retroperitoneal fibrosis and pancreatitis. Ocular involvement most commonly includes chronic lid swelling and proptosis, but may include visual disturbances from orbital apex syndrome.12
Both Tolosa-Hunt syndrome and immunoglobulin G4-related disease have characteristic findings on neuroimaging and generally respond to steroids.
Vasculitides associated with antineutrophil cytoplasmic antibody (ANCA) (e.g., granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis) generally present with pulmonary, gastrointestinal and neurological involvement but can also have ocular involvement like orbital apex syndrome, as well as corneal ulceration, episcleritis, scleritis and retinal vascular occlusion.
Sarcoidosis is a granulomatous inflammatory disease that primarily affects the lungs. The most common ocular involvement of sarcoidosis is anterior uveitis, although cases of sarcoid granulomas eroding into the orbital apex have been reported.13
Because immunosuppressive therapy is indicated for inflammatory causes, infectious etiologies should first be considered so as not to exacerbate them with treatment.
Bacterial and fungal infections of the paranasal sinuses can spread to the contiguous orbital apex. Fungal infections are primarily found in immunocompromised patients.
Neoplastic etiologies include meningeal infiltration of leukemia or lymphoma, as well as nasopharyngeal carcinoma, which portends a poor prognosis.14
A serologic vasculitis workup including ANCA, antinuclear antibodies and double-stranded DNA antibodies was negative. Human immunodeficiency virus testing was negative. Lumbar puncture was performed to evaluate for evidence of inflammatory, infectious and neoplastic processes in the central nervous system. Cerebrospinal fluid analysis showed no white blood cells, normal protein, elevated glucose (122 mg/dL, reference range 45–80 mg/dL), and no growth on cultures.
4. What is the preferred imaging method for evaluating lesions of the orbital apex?
High-resolution MRI with and without contrast is preferred for evaluating most lesions of the orbital apex.8 MRI provides superior soft-tissue contrast compared with other imaging methods, and orbital fat suppression can also improve lesion visibility.15 A short-tau inversion recovery image or T2-weighted fat-suppressed series can be included to evaluate for inflammatory edema and purulent fluid collections.16
CT is useful for evaluating bone involvement at the orbital apex, especially in the setting of trauma.10 It is also used for patients with magnetic foreign bodies, surgical clips or other MRI contraindications.
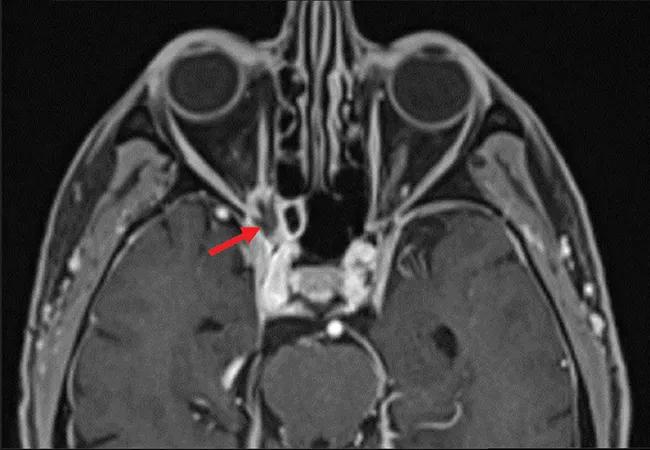
Brain MRI revealed an irregularly shaped lesion with peripheral enhancement and a central nonenhancing region just inferior to the right optic nerve at the orbital apex (Figure 2). It was thought that this finding might represent a small abscess or area of necrosis. Scattered paranasal sinus mucosal thickening and increasing asymmetric enlargement of the right anterior cavernous sinus relative to previous images were also noted.
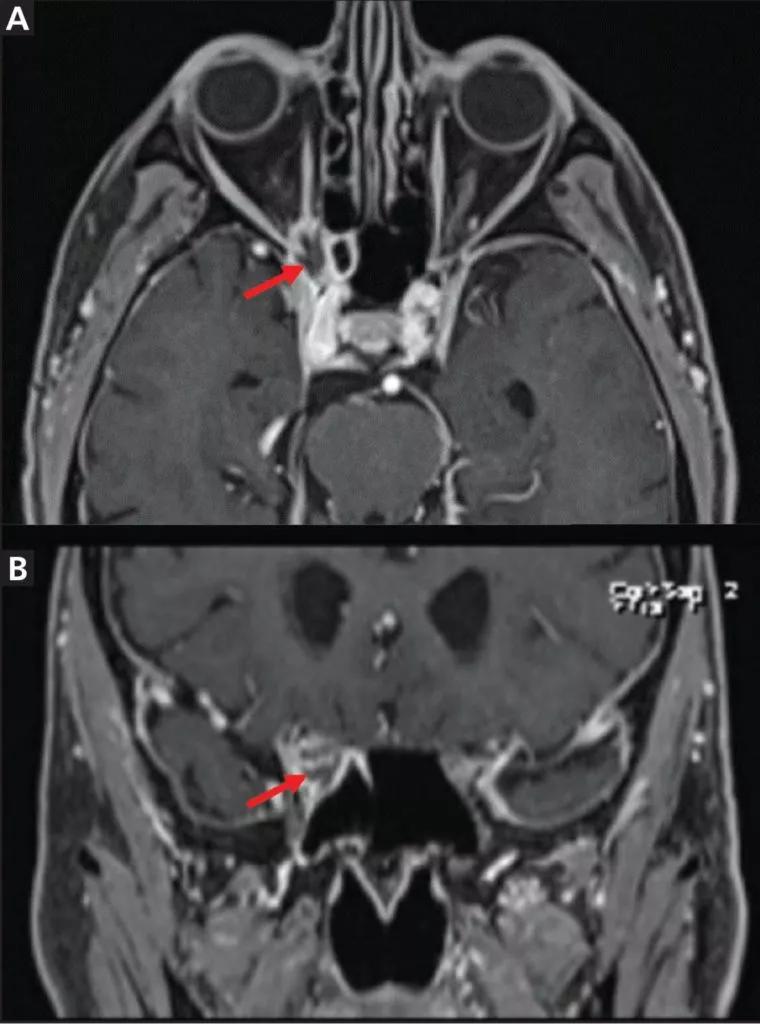
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fed9d11f-a57d-4ec2-8b55-cf3cb8260d92/F2_large_-760x1024_jpg)
Figure 2. Magnetic resonance imaging of the orbit showed lesions (arrows) in axial T1-weighted fat-suppressed series (A) and coronal multiplanar reformation (B) views.
Sinus CT showed corresponding heterogeneous soft tissue at the right orbital apex with smooth bony remodeling and subtle erosive changes, raising suspicion for a neoplasm, infection or an inflammatory entity. There appeared to be thinning and convexity of the right sphenoid sinus roof in addition to erosion of the right optic strut and along the inferior margin of the right anterior clinoid process.
An endoscopic transnasal intracranial biopsy was performed. An incision made within the right orbital apex inferior to the optic nerve returned purulent material. Frozen sections from the right orbital apex were negative for neoplasm but showed invasive fungal hyphae within fragments of fibrous tissue with focal necrosis. On Grocott-Gomori methenamine silver stain, the organism morphologically resembled aspergillus species. Aspergillus fumigatus was confirmed by fungal culture.
The patient was then diagnosed with invasive orbital aspergillosis with involvement of the orbital apex and cavernous sinus.
5. Which pair of organisms most commonly cause orbital mycoses?
Aspergillosis and mucormycosis are the most common causes of orbital fungal infections.17
Candida species are the most common etiologic pathogens of keratitis. Fusarium species are also one of the predominant causes of corneal fungal infections and are the most likely fungal pathogen to cause infection following eye trauma.18
Orbital mycoses are most often a result of contiguous spread from the paranasal sinuses.10
Rhizopus species are the most common cause of mucormycosis, which is classically associated with diabetes.19,20
A flavus and A fumigatus are the most common species of aspergillus that affect the orbit.21 Orbital aspergillosis presents in invasive and noninvasive forms.
Noninvasive aspergillosis typically does not display fungal infiltration of tissue but can produce a thick material known as allergic mucin along with extramucosal mycotic proliferations known as fungus balls, which are seen primarily in patients who are immunocompetent. If located in the posterior sphenoid or ethmoid sinus, a large fungus ball may compress the optic nerve and cause vision loss and even orbital apex syndrome.22
Invasive aspergillosis is characterized by bone invasion and fungal tissue that behaves similarly to an inflammatory or malignant process.23 Locally, there is invasion of nearby structures and blood vessels, causing thrombosis and tissue necrosis. In the fulminant form of invasive aspergillosis, there is embolization and multiorgan involvement, potentially leading to death.21 Risk factors for invasive aspergillosis include total neutrophil count of less than 1,000/mm3, T-cell defects (e.g., from human immunodeficiency virus), defective phagocytosis, hematologic malignancy, immunosuppressive agents, diabetes mellitus, prosthetic devices, trauma, excessive environmental exposure, residence in an endemic area (e.g., Sudan) and advanced age.20 While incidence of invasive disease is much greater in immunocompromised patients, cases have also been reported in immunocompetent hosts.21-30
6. Which antifungal medication is preferred for the initial treatment of invasive aspergillosis?
The Infectious Diseases Society of America 2016 update of practice guidelines for diagnosis and management of aspergillosis recommends voriconazole for initial medical therapy for invasive sinus aspergillosis.30 For patients who are intolerant to voriconazole, the best alternative is a lipid formulation of amphotericin B or isavuconazole. Treatment is recommended for a minimum of six to 12 weeks, depending on the degree of immunosuppression, infection location and evidence of improvement.
Lipid formulations of amphotericin B are less likely to cause nephrotoxicity compared with amphotericin B deoxycholate. Amphotericin B deoxycholate is not recommended for use in invasive aspergillosis unless lipid formulations of amphotericin or other mold-active antifungals (such as voriconazole) are unavailable.30 Hyperbaric oxygen and retrobulbar amphotericin B injections are less commonly used to treat orbital mycoses, but there is some evidence for their viability.31-33 Retrobulbar injections may be useful if aggressive orbital debridement is not favored or if the burden of orbital disease is not substantial.33
In general, combination therapy is not recommended, but the use of voriconazole with an echinocandin may be considered for patients with severe disease, hematologic malignancy or profound persistent neutropenia.30
In addition to medical therapy, surgical debridement is usually required and may involve exenteration (i.e., surgical removal of the entire globe and surrounding structures) in cases of orbital apex involvement.22,30
Invasive aspergillosis carries a significantly worse prognosis than the noninvasive form. Invasion of bone and blood vessels makes surgical access and drug penetration challenging and allows the fungus to spread intracranially. The reported mortality rate associated with invasive aspergillosis is 40%, rising to 50% when there is central nervous system involvement.34
Prognosis is often worsened by initial misdiagnosis owing to presenting features that are largely nonspecific. Initial administration of corticosteroids is also associated with a poorer prognosis as a result of iatrogenic potentiation of the infection.17
The patient was started on voriconazole and tapered off prednisone. CT of the chest was performed to investigate the lungs for additional areas of infection and was negative. Oculoplastic surgery was consulted regarding the benefit of right orbital exenteration. Because the infection had already spread to the cavernous sinus, it was determined that exenteration would not improve survival and was therefore deferred.
Three weeks after discharge, the patient continued to experience retro-orbital headaches but rated the pain as 1/10 instead of 10/10, as he had consistently rated it before treatment. His right-sided ptosis was slightly improved, but his right eye blindness, afferent pupillary defect and complete loss of extra-ocular movements persisted.
For a list of references, see the original article in Cleveland Clinic Journal of Medicine (2021;88[9]:494-501).
Dr. Nielsen is staff in the Department of Internal Medicine at Cleveland Clinic. Dr. Bernstein is an internal medicine resident at Cleveland Clinic. Ohlhausen is a medical student at Case Western Reserve University School of Medicine.
Advertisement

Researchers explore the mental and physical benefits of social prescribing

Multidisciplinary approach helps address clinical and psychosocial challenges in geriatric care

Effective screening, advanced treatments can help preserve quality of life

Study suggests inconsistencies in the emergency department evaluation of geriatric patients

Auditory hallucinations lead to unusual diagnosis

How providers can help prevent and address this under-reported form of abuse

How providers can help older adults protect their assets and personal agency

Recognizing the subtle but destructive signs of psychological abuse in geriatric patients