Outcome benefits from large registry lay groundwork for a randomized controlled trial
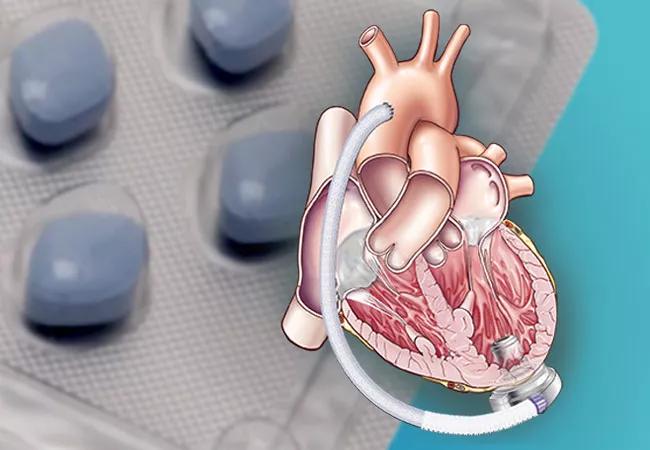
Patients with an implanted contemporary centrifugal left ventricular assist device (LVAD) who received phosphodiesterase-5 inhibitor (PDE-5i) therapy after implant had lower rates of death and ischemic stroke than comparable patients not taking a PDE-5i. This finding, from a retrospective analysis of more than 7,000 patients in the national STS (Society of Thoracic Surgeons) Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), was published in JACC: Heart Failure by a team of Cleveland Clinic investigators.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“This study provides intriguing preliminary evidence that adjunctive PDE-5i therapy could provide additional benefits for patients with a contemporary LVAD,” says heart failure cardiologist Randall C. Starling, MD, MPH, the study’s corresponding author. “If these results are confirmed in a randomized controlled trial, we expect that PDE-5i therapy will become standard of care in this setting.”
PDE-5is have multiple effects that, in theory, could improve outcomes for patients with an LVAD. They have antiplatelet and antithrombotic effects, facilitate right ventricle unloading in patients with LVADs and have been shown experimentally to improve right ventricular contractile function.
The PDE-5i sildenafil, which is FDA-approved for erectile dysfunction and pulmonary arterial hypertension, has drawn increasing interest as a possible treatment for cardiovascular disease, with encouraging results. It has not, however, been studied in a randomized controlled trial for patients with an LVAD.
A previous observational study from the current study’s investigators (J Am Heart Assoc. 2020;9[14]:e0158897), based on earlier STS INTERMACS registry data, found fewer thrombotic events and improved survival in patients with an LVAD if they were receiving a PDE-5i. However, that study’s cohort consisted mostly of patients with a HeartMate IITM LVAD, an axial-flow device that is no longer implanted.
Over the years, LVADs have evolved from the first-generation pulsatile-flow devices to continuous-flow devices, including the second-generation (axial flow) and third-generation (centrifugal flow) pumps.
Advertisement
The third-generation HeartMate 3TM is currently the only durable LVAD approved for use in the U.S. Although associated with improved stroke and mortality profiles, it is still associated with a 10% risk of stroke over 24 months and a 75% event-free survival rate at 24 months, leaving room for continued improvements with effective adjunctive therapy.
The current study included 7,229 patients registered in STS INTERMACS between September 2017 and March 2020. All had been implanted with a continuous-flow centrifugal LVAD and had data regarding pharmaceutical treatment. Patients were taking standard anticoagulation and antiplatelet therapies.
The devices were a HeartMate 3 (n = 4,628) and HeartWareTM VAD (n = 2,601). The HeartWare VAD has since been removed from the market by its vendor.
Of the total cohort, 2,173 patients (30.1%) were taking a PDE-5i post-implant. Propensity matching was used to adjust for baseline differences between patients who were and were not taking a PDE-5i. Dr. Starling notes that the use of PDE-5i therapy by patients in the registry is not supported by clinical trial evidence that it improves outcomes in patients with an LVAD and that such therapy is usually given because the patient has pulmonary hypertension.
The primary endpoint — a composite of all-cause mortality, ischemic stroke or pump thrombosis — occurred in:
This translates to lower risk for patients taking a PDE-5i (adjusted hazard ratio [HR] = 0.77; 95% CI, 0.69-0.86; P < 0.0001). Results were similar whether patients had received a HeartMate 3 or HeartWare device.
Advertisement
The following were the study’s secondary endpoints:
PDE-5i use was associated with increased gastrointestinal bleeding (adjusted HR = 1.18; 95% CI, 1.04-1.34; P = 0.01).
“This study’s results with the newer LVADs are consistent with findings from our prior study that analyzed patients with the obsolete models,” observes study co-author Edward Soltesz, MD, MPH, Surgical Director of Cleveland Clinic’s Kaufman Center for Heart Failure Treatment and Recovery. “It adds support to the idea that adding PDE-5i therapy merits a randomized clinical trial.”
Although the evidence for benefit of PDE-5i use in patients with a contemporary centrifugal LVAD is growing, the authors note that the study was limited by being a registry-based, nonrandomized, observational study. Furthermore, dosage, type and duration of PDE-5i therapy were unavailable from the registry.
“Cleveland Clinic is actively exploring initiating a multicenter randomized trial for patients with a contemporary LVAD,” Dr. Starling concludes. “This is critical before PDE-5is can be endorsed for this use.”
Advertisement
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more