As vaccine availability increases, expect to see more complaints of palpable masses and abnormal results from routine breast screening
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c5f52bdb-5eb8-49ff-b441-b3f36c494066/CCC-2073110_03-03-21_007_SG-jpg)
COVID-19 vaccines
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
A 32-year-old woman is referred to breast radiology by her primary care provider for evaluation of palpable lumps in the left axilla. Ultrasound of the area shows multiple enlarged lymph nodes with cortical thickening in the left axilla. She reports a COVID-19 vaccine in the left arm 10 days prior. The patient’s unilateral axillary adenopathy is likely a reaction to the vaccination; however, short-term follow-up is warranted. The patient is asked to return to radiology in 4-12 weeks, at which point we will reevaluate. If the unilateral axillary adenopathy persists at follow-up, we would consider lymph node sampling to rule out malignancy.
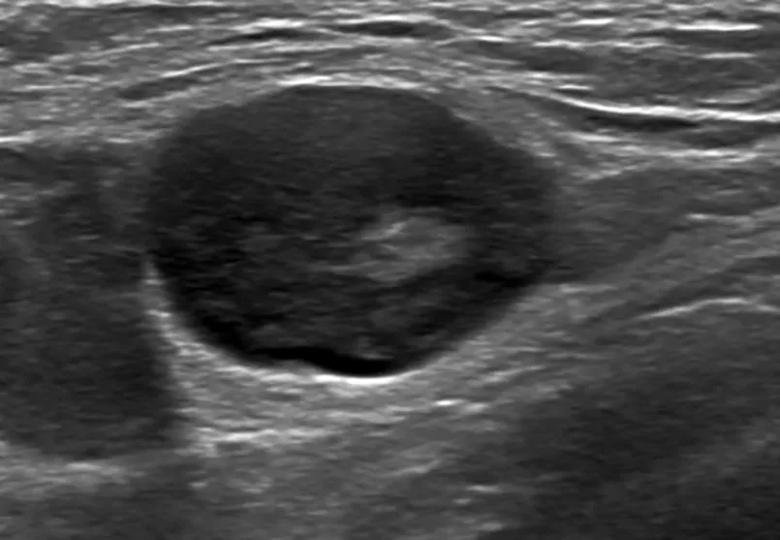
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/51832aa8-38d3-466a-908e-7981863c5774/21-WHI-2064882_CQD_inset-805x557-1_jpg)
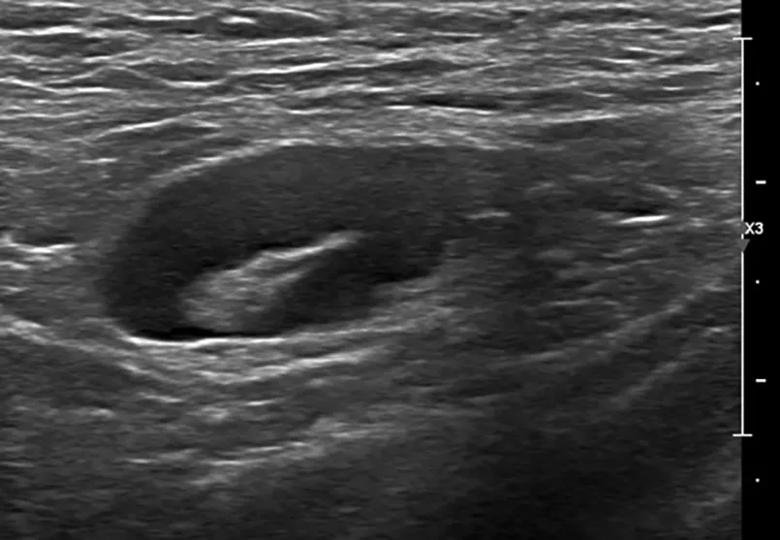
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a692d5e2-91cc-495d-afe5-b0bcc802b361/21-WHI-2064882_CQD_inset-805x557_2_jpg)
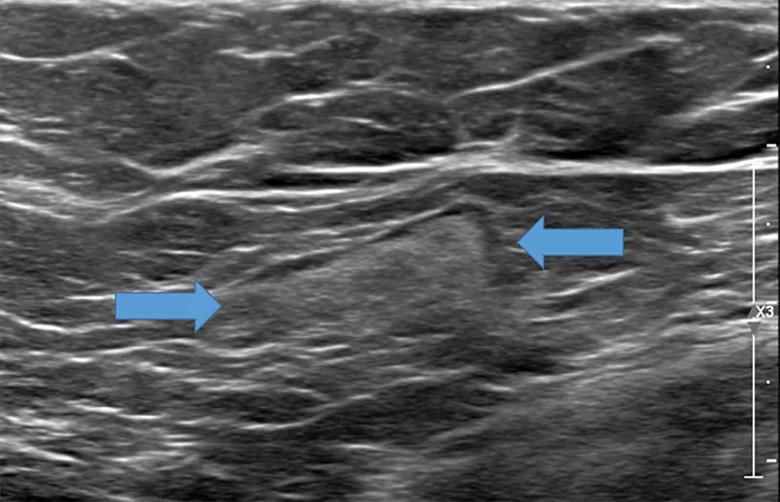
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/e4313c89-d53e-4a7d-a933-762b3832199a/21-WHI-2064882-CQD-inset_805x518_jpg)
A small, but not insignificant number of people develop unilateral axillary adenopathy in the days following vaccination against SARS-CoV-2, the virus that causes COVID-19.
In general, any vaccine could cause this sort of reactive lymph node enlargement. Looking back, this phenomenon was documented occasionally as a reaction from other vaccines, including measles, smallpox and anthrax. Lymphadenopathy is an expected part of a healthy immune response. That said, this adenopathy seems much more robust with our current COVID-19 vaccines than in historical vaccines.
Results from the Moderna trials demonstrate that, following the second dose, axillary adenopathy occurred in 16% of women aged 18-64 years. The incidence reported by Pfizer is less as researchers only collected unsolicited axillary symptoms; however, the Pfizer data does provide insight into the time course of lymphadenopathy: generally it was reported 2-4 days following vaccination and the mean duration was 10 days.
Advertisement
Lymph node abnormality is an uncommon finding in screening breast exams. Specifically, we only see lymph node abnormality in about 0.02%-0.04% of screening mammograms. As COVID-19 vaccinations become more readily available to the broader population, we expect to have more patients reporting these palpable masses or with axillary adenopathy on mammogram.
After identifying axillary adenopathy on a screening mammogram, further assessment of the ipsilateral breast is warranted, and should include a full medical history (including information about whether or not the patient received the COVID-19 vaccine, which vaccine they received, when, and in which arm). The Society for Breast Imaging currently recommends a short-term follow-up exam 4-12 weeks following the second vaccine dose to rule out malignancy.
At Cleveland Clinic, one of the first things we did in response to this was to amend our patient intake form. Now, in addition to asking for a personal breast cancer history and family history, we also ask if the patient has had a recent COVID-19 vaccine injection. We document the manufacturer, date of injection, and the arm in which the patient received it. Currently, for every screening mammogram I review, there’s a note from the technologist that says: “COVID-19 vaccine? Yes/No.”
If we see lymphadenopathy, we perform ultrasound to establish a baseline, and then bring the patients back several weeks after the second dose to compare the imagining to ensure that the nodes have returned to normal.
Advertisement
It’s important for physicians to stress that screening mammograms should not be postponed. Asymptomatic patients seeking routine screening may wish to schedule their mammogram 4-6 weeks following the COVID-19 vaccine. However, we do not want to discourage patients from receiving the vaccine. If patients are offered the opportunity for vaccination, they should take it. Lymphadenopathy does not occur in a majority of patients, and if it does, we will follow up as needed.
Last year, many patients postponed important screenings because of the pandemic. Another message we need to be sharing widely with our patients is that it is safe to have a mammogram. We have a year of experience providing healthcare during a pandemic in a way that keeps our patients and caregivers safe. We have universal masking, health screenings at each entrance and physical spacing throughout our facilities. Yes, it is safe to seek care.
About Dr. Dean:Laura Dean, MD, is a diagnostic radiologist who specializes in breast imaging.
Advertisement
Advertisement
Goal-of-care discussions drive earlier hospice access
Clinical trials and de-escalation strategies
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting