Long-acting antiemetics and high-dose steroids key to minimizing acute nausea
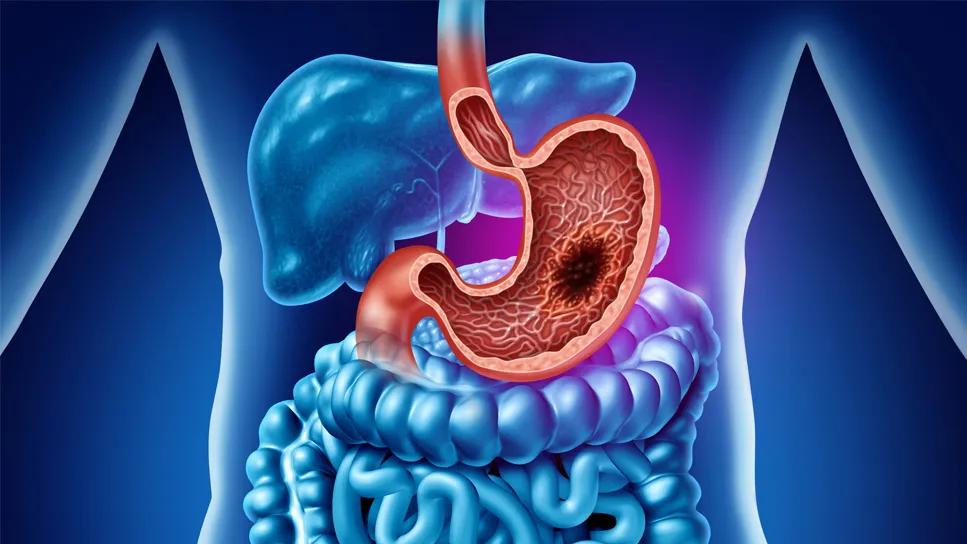
The prognosis for metastatic gastroesophageal (GE) cancer is poor, with an average survival of about one year. A newly FDA-approved targeted therapy, zolbetuximab, improves outcomes for patients whose tumor express the protein CLDN18.2.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In many gastric and gastroesophageal junction cancers, CLDN18.2 is overexpressed, causing tumors to grow and proliferate. In October 2024, the FDA approved zolbetuximab, a CLDN18.2-directed monoclonal antibody to be used with fluoropyrimidine- and platinum-containing chemotherapy in the treatment of certain types of gastric cancer and gastroesophageal junction adenocarcinoma.
The IV therapy is approved for first-line treatment in adults with locally advanced tumors that are unresectable or metastatic human epidermal growth factor receptor 2 (HER2)-negative gastric or gastroesophageal junction (GEJ) adenocarcinomas that are CLDN18.2-positive.
In two large randomized, phase 3 trials (GLOW and SPOTLIGHT), giving zolbetuximab alongside FOLFOX (leucovorin calcium, fluorouracil and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) chemotherapy resulted in a longer median overall survival of 2.5 to 3 months over chemotherapy alone. “In this tumor type, for patients with very advanced disease, that’s clinically meaningful,” says Cleveland Clinic GI Oncologist Suneel Kamath, MD. “This is exciting because gastric and gastroesophageal cancers have mostly had chemo-only treatment for a long time. We need more biomarker-targeted therapies.”
Although there have been some targeted therapies approved for GE cancers previously, the biomarkers involved are often less common. Since CLDN18.2 is highly expressed in roughly 35% of patients, this opens up the opportunity for biomarker-directed therapy for many patients.
Advertisement
Dr. Kamath notes the importance of educating patients and providers about this new treatment. Since GE cancers are somewhat rare, some community cancer centers may be less familiar with the current treatment landscape and may not be as aware of this new targeted therapy.
This new approval places greater importance on biomarker testing for GE tumors prior to treatment initiation. CLDN18.2 can be found through standard immunohistochemistry (IHC) tests.
Only an estimated 60-70 percent of patients with GE cancers currently receive complete biomarker and genomic testing, and many of these tests didn’t previously include CLDN18.2. “It’s particularly important that even if a patient had genomic or next-generation sequencing (NGS) testing done previously, that CLDN18.2 is added to determine if your patient may be eligible for zolbetuximab,” says Dr. Kamath.
Dr. Kamath cautions that although this targeted therapy is an advancement over the standard chemo backbone, its side effects need to be managed aggressively. In the clinical trials, many patients experienced significant acute nausea, sometimes during the infusion itself.
Longer-lasting agents such as palonosetron and granisetron may help mitigate these side effects. Neurokinin-1 receptor blockers such as fosaprepitant can also help stave off nausea. Dr. Kamath notes that in the trials, there was significant variability in the amount of antiemetic pre-medication given to patients at different cancer centers, which may have contributed to the higher rates and severity of nausea seen in the trials.
Advertisement
“We need to be very proactive about preventing these side effects. It's possible that by premedicating patients more aggressively, we can improve side effects compared to what was seen in the clinical trials,” says Dr. Kamath.
Cleveland Clinic Cancer Institute has added zolbetuximab to its formulary and it is available to patients at all of its oncology clinics. The clinical team established a treatment plan to ensure strong anti-nausea medications and other supportive tools are available for patients.
The medication can be given in a two-week regimen with FOLFOX or a three-week regimen with CAPOX.
Zolbetuximab is now being studied in both the adjuvant and neoadjuvant settings. Researchers are also investigating whether there is synergy between combining the treatment with chemotherapy and immunotherapy in a way that is safe and tolerable.
Advertisement
Advertisement

Microvascular “supercharging” is a critical newer step to promote favorable outcomes
Dysphagia and SUVmax are predictive, but other suspected factors are not

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer