Regenerative medicine one day may offer alternatives to joint surgery
A biologic therapy shown to reduce inflammation in surgical and wound care may do the same when injected into joints with osteoarthritis. This treatment, developed from amniotic membrane and umbilical cord tissue, already has been cleared for some indications by the U.S. Food and Drug Administration but is now being tested in a randomized phase 2 trial studying its efficacy in knee osteoarthritis compared to placebo.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“We think this could be a landmark study,” says orthopaedic surgeon Alan Davis, MD, who is Cleveland Clinic’s principal investigator in the multisite study. “I have been using the product since the 1990s as graft tissue to modulate inflammation around nerves and tendons in foot and ankle surgery. It has provided great results for my patients. Now I’m excited to see what it might do when injected into arthritic knee joints.”
The trial is one of three at Cleveland Clinic currently investigating injectable biologic therapies for osteoarthritis.
“Our Joint Preservation Center provides the communication, education and resource development that enables Cleveland Clinic clinicians and scientists to step forward to participate in and lead trials like these,” says orthopaedic surgeon George Muschler, MD, who heads the center. “All of our orthopaedic providers work together to give patients access to and awareness of the safest and most effective biologic therapies for joint disease.”
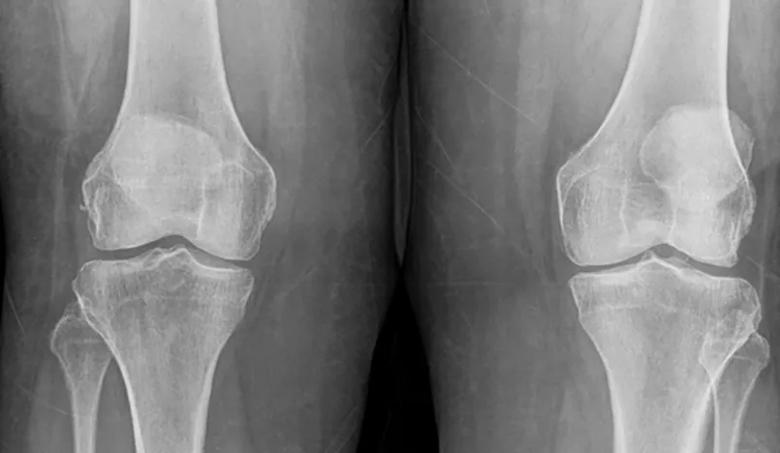
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/67b461b5-697d-46d5-ad1a-3da6acca357b/22-ORI-3272723-CQD-Inset-2_jpg)
X-ray of a patient with early osteoarthritis, illustrating the state of disease when injection therapies may be most effective.
Dr. Muschler also directs the Bone Biology Laboratory within Cleveland Clinic’s Lerner Research Institute. His lab, funded primarily by the National Institutes of Health as well as philanthropy, focuses on developing improved methods of tissue engineering and regenerative medicine.
“Today, regenerative medicine is focused on preservation and restoration of cartilage, but also bone and heart, retina, brain, kidney, lung and liver tissue. It is even being applied to cancer therapy,” says Dr. Muschler. “The implications extend far beyond what we can currently envision. We are barely scratching the surface.”
Advertisement
In the study led by Dr. Davis, participants are between ages 35 and 85, with moderate-to-severe arthritis of the knee (Kellgren-Lawrence grade 3-4) that has not responded to conservative therapies (e.g., anti-inflammatory medication, weight loss, physical therapy, bracing) or other injections, such as corticosteroids and hyaluronic acid. At screening, participants’ Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain is 70 or higher, contributing to a decreased quality of life.
Participants are randomly assigned to receive a single ultrasound-guided intra-articular injection of either the investigative therapy or saline. Primary outcomes are pain relief and functional improvement (using a KOOS score for pain, physical function and quality of life, and other tools) at 12 weeks. Participants return for regular follow-up exams for a full year after injection.
“We will evaluate patients’ gait and range of motion and assess pain levels — if patients can do more activities after the injection than they could before,” says Dr. Davis. “The investigational product, made from birth tissue, is believed to have inflammatory modulating or anti-inflammatory properties. If we can reduce pain and improve function, it is hoped the product may translate into the option to delay or avoid other surgery, such as joint replacement.”
In another study, Cleveland Clinic Florida researchers are evaluating the effects of injected bone marrow aspirate concentrate (BMAC) compared to traditional corticosteroid injections in patients with hip osteoarthritis. BMAC has shown promise in improving pain and function in knee osteoarthritis. This is the first randomized controlled trial in the hip.
Advertisement
“In my own clinical experience, we have seen a diminished response in patients receiving repeated corticosteroid injections (e.g., triamcinolone, cortisone). In fact, the latest research suggests that long-term use of steroids may actually accelerate joint deterioration,” says the study’s principal investigator Leonardo Oliveira, MD, a sports medicine physician at Cleveland Clinic Florida. “There is clearly a need for safer and more effective nonsurgical treatment alternatives.”
Study participants are between ages 18 and 65, with hip osteoarthritis Kellgren-Lawrence grade 2 or higher. At baseline, patients must report a pain score of 4 or higher on the Western Ontario McMaster Universities Arthritis Index (WOMAC), despite completing physical therapy in the previous six months.
Study participants receive a single ultrasound-guided injection of either BMAC or triamcinolone. BMAC therapy starts with aspiration of a patient’s own bone marrow from their pelvis using a needle. The marrow is then processed to remove red blood cells, while concentrating nucleated cells, platelets, growth factors and cytokines. Patients receiving triamcinolone have a simulated aspiration procedure.
“BMAC will contain some stem cells, but it is not a ‘stem cell therapy,’” says Dr. Muschler. “Stem cells are the least common cell of all the cells injected using BMAC. For this reason, we use the term ‘cellular therapy.’”
Researchers will track the change in patient-reported pain and physical function according to the WOMAC, as well as other health data, over 12 months.
Advertisement
“We hypothesize that the BMAC will produce longer-term pain relief, while triamcinolone will produce quicker but less durable pain relief,” says Dr. Oliveira.
In a third study, researchers at Cleveland Clinic and other medical centers are studying the efficacy of hyaluronic acid combined with platelet-rich plasma (PRP) compared with hyaluronic acid alone or placebo for the treatment of knee osteoarthritis.
“Many studies have suggested that PRP improves symptoms of knee osteoarthritis without serious adverse events,” says primary investigator Nicolas S. Piuzzi, MD, Director of Adult Joint Reconstruction Research in Cleveland Clinic’s Department of Orthopaedic Surgery. “In our current study, we are investigating the benefit of combining PRP with hyaluronic acid, especially in patients who still have knee pain after injection with hyaluronic acid alone.”
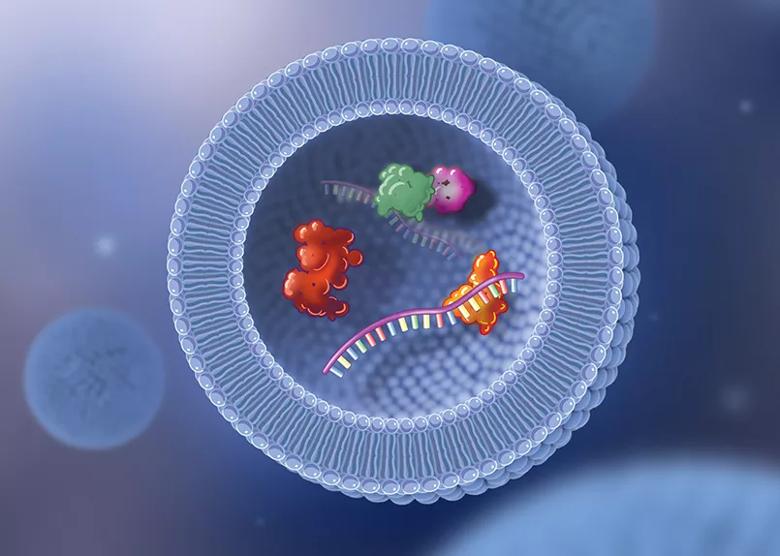
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5bd7ff15-ab89-4940-995c-488493e7be2c/22-ORI-3272723-CQD-Inset-3_jpg)
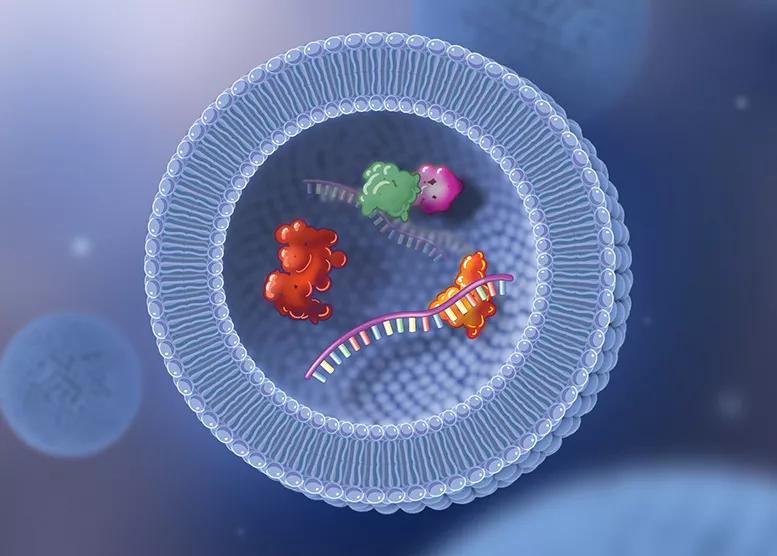
Illustration of an exosome. When platelets from a patient’s blood are concentrated and injected into a joint, there is a transfer of exosomes that are released from those platelets. Exosomes are very small, fatty, droplet-like particles that can contain various biologically active proteins and cytokines, as well as microRNA, which when released provides signals to other cells. The transfer of exosomes is one of the mechanisms being considered to explain the anti-inflammatory effect and clinical benefits of PRP injection therapy.
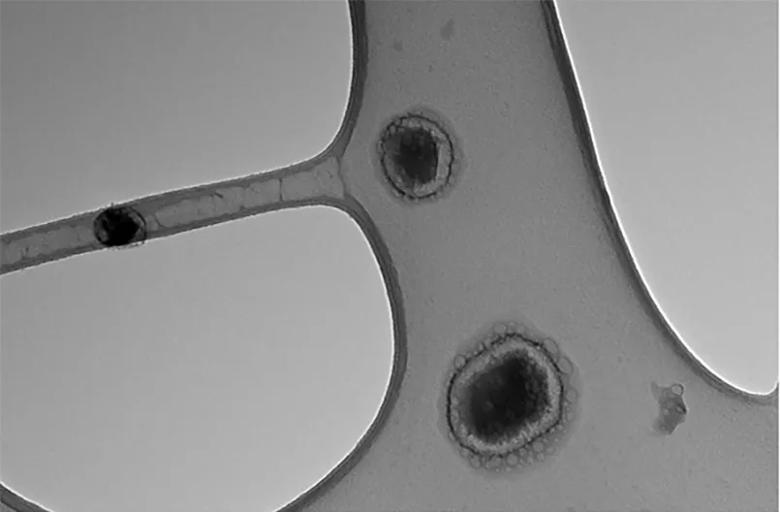
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/9cf5d580-acd4-4ac1-8534-bf88ac8f9e6f/22-ORI-3272723-CQD-Inset-1_jpg)
Exosomes viewed through a transmission electron microscope.
Study participants are between ages 50 and 80, with Kellgren-Lawrence grade 2 or 3 knee osteoarthritis and significant unilateral knee pain. They are randomly assigned to one of the three treatment/placebo arms. Primary outcomes are the changes in pain and function (according to WOMAC scores) six months after the injection.
“An earlier French study showed that 90% of patients who still had pain after hyaluronic acid injection reported improvement in pain and/or physical function after having another injection combined with PRP,” says Dr. Piuzzi. “I am excited to see how Cleveland Clinic and other patients respond to this investigational therapy.”
Advertisement
In the very short term, these studies will determine if we already have minimally invasive injection therapies that can reliably delay or prevent the need for joint replacement surgery, explains Dr. Muschler.
“These studies show that our teams at Cleveland Clinic are committed to testing the safety and efficacy of new biologic therapies, educating our patients about choices, improving patients’ health, and advancing knowledge to serve future generations,” he says. “We are learning who benefits from new therapies, and how much. Just as important, we are learning who does not benefit, so that these patients can be informed, avoid false hope and wasted effort, and be recruited into the process of finding something better.”
Advertisement

Nonthermal technique reduces bleeding and perforation risk

Standardizing a minimally invasive approach for Barrett’s Esophagus and Esophageal Cancer

PSMA-targeted therapy for metastatic prostate cancer now offered at Cleveland Clinic Weston Hospital

Nationally recognized urologic oncologist offers vision for growth, innovation, and excellence

Noninvasive modality gains ground in United States for patients with early-to-moderate disease

Cleveland Clinic Weston Hospital’s collaborative model elevates care for complex lung diseases

Interventional pulmonologists at Cleveland Clinic Indian River Hospital use robotic technology to reach small peripheral lung nodules

Trained in the use of multiple focal therapies for prostate cancer, Dr. Jamil Syed recommends HIFU for certain patients with intermediate-risk prostate cancer, especially individuals with small, well-defined tumors localized to the lateral and posterior regions of the gland.