Cleveland Clinic Cancer Institute among select group of centers to administer highly personalized treatment
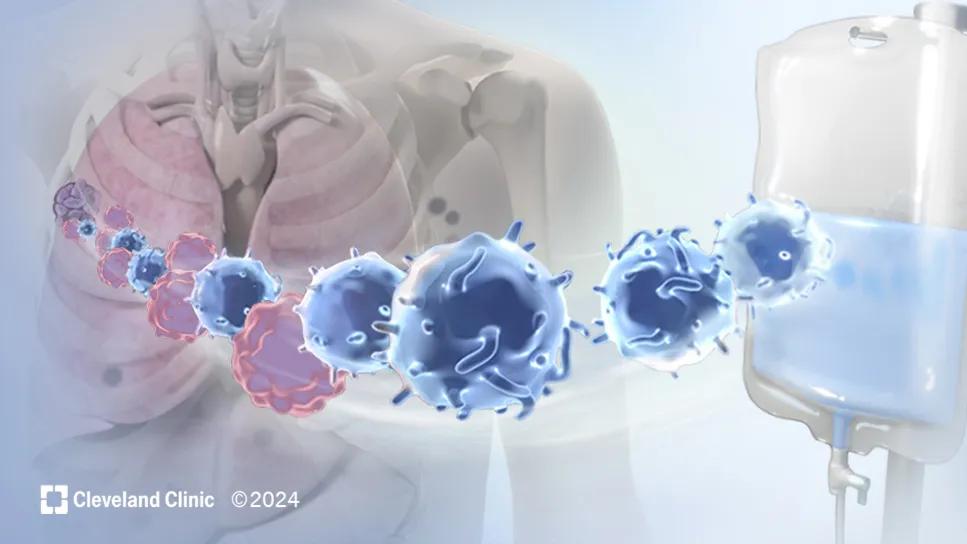
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/34df49ca-7f47-45b0-aa65-6f70cb1f741d/truong-til-therapy-medical-illustration)
Tumor-Infiltrating Lymphocytes (TIL) therapy
When the FDA granted accelerated approval of tumor-infiltrating lymphocyte (TIL) treatment with lifileucel for patients with metastatic melanoma, Cleveland Clinic Cancer Institute was one of 23 select Early-Authorized Treatment Centers across the country approved to administer this new form of cellular therapy. This is the first-of-its-kind treatment for solid tumor malignancy. This autologous cell therapy is approved for adult patients with unresectable or metastatic melanoma who have previously been treated with a PD-1 inhibitor, or a BRAF inhibitor for those who are BRAFV600 positive.
“This is a major advancement in the cancer treatment landscape," says James Isaacs, MD. “This process uses a patient's own immune cells to make a personalized cell therapy that can find and attack the cancer cells.”
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
TIL therapy leverages the body's own T lymphocytes that are in contact with a harvested tumor site to seek out and destroy other tumors cells and targets. TIL cells recognize and attack tumor cells. However, it requires many of these cells to do the job. The goal is to make enough cells for the therapy to succeed.
The process of TIL therapy begins with a surgeon removing a melanoma tumor from the patient the size of 1.5-4 centimeters. Immune cells from the tumor are then isolated and extracted in a lab and made into an infusion that the manufacturer ships to the patient’s treatment team. After this process, the patient receives a lymphodepleting chemotherapy regimen consisting of cyclophosphamide, mesna and fludarabine to prepare their body to accept the TIL cells that will be infused.
Within 24 hours of the lymphodepleting chemotherapy, the generated TIL cells are infused back into the patient, where they then work to find and destroy the melanoma tumor cells.
Video content: This video is available to watch online.
View video online (https://cdnapisec.kaltura.com/p/2207941/sp/220794100/playManifest/entryId/1_jcwy5l4x/flavorId/1_5f3sgelj/format/url/protocol/https/a.mp4)
Currently, checkpoint inhibitor therapy has the highest efficacy as frontline treatment for melanoma, at roughly 40-60%, depending on the regimen chosen. One challenge with standard immunotherapy is that although it can activate immune cells, it isn’t always able to direct them to the tumor(s). Immune checkpoint inhibitors also have the potential to aberrantly activate the immune system, not just against cancer but against the patient themselves. This results in autoimmune side effects.
TIL therapy overcomes some of these limitations by utilizing the patient’s own immune system directly. "The beauty of TIL therapy is that the cells were the patient’s to begin with,” says Dr. Isaacs.
Until now, treatment options were limited when frontline treatment for melanoma did not achieve a sufficient response. FDA approval of TIL therapy was based on a worldwide, multicenter trial of patients with unresectable or metastatic melanoma who had been treated with at least one line of systemic therapy. Of the 73 patients treated in the study, there was an overall response rate of 31.5%.
The goal of this one-time treatment is to achieve a durable response without having to remain on treatment on an ongoing basis.
Advertisement
Currently, TIL therapy is approved for standard use only in the U.S. As a center with a specialized high-dose chemotherapy inpatient service and a high-volume melanoma service, Cleveland Clinic Cancer Institute was selected as one of 23 Early-Authorized Treatment Centers to administer this personalized cell therapy.
In terms of eligibility, patients need to have a tumor that is at least 1.5-centimeters in size, and they need to be fit enough to tolerate high-dose chemotherapy at doses similar to those given prior to bone marrow transplant.
It takes roughly six weeks for TIL cells to be manufactured and be ready for reinfusion, so making referrals for TIL therapy early is advisable.
"If a patient is on standard immune therapies and is not responding after the first few doses, it may be time to consider TIL therapy as an option," says Dr. Isaacs. "I always encourage early communication. We are happy to visit with patients to review the TIL process and determine if it is the right time to move forward with the treatment."
Since TILs are made from each patient and are thus a personalized treatment, side effects tend to be short term and related to the supportive treatments given rather than the TILs themselves. Typical side effects are those commonly related to chemotherapy, such as chills, fatigue, pyrexia, edema and infection. These do need to be monitored carefully, which is why patients remain in the hospital for roughly four to six weeks after receiving treatment.
The current versions of TIL therapy do require high doses of chemotherapy and an inpatient hospital stay. "There is a lot of ongoing research with an effort to improve the TIL therapy regimen," says Dr. Isaacs. "We are hopeful that eventually less intensive pre-treatment will be used, and we aim to move these treatments to an outpatient or clinic setting with less hospital time."
Advertisement
Trials are underway for TIL therapy in patients with other cancers as well as studies of other TIL products. Researchers believe that this treatment strategy is likely to benefit patients with a wide range of solid tumors. Cleveland Clinic Cancer Institute is leading the only current research study of TIL therapy after curative surgery.
Advertisement
Advertisement
Collaborative patient care, advanced imaging techniques support safer immunotherapy management
Highly personalized treatment shrinks tumors resistant to immunotherapy
Researchers develop first-of-its-kind neoantigen atlas to better understand immunotherapy resistance
Patients receive specialized inpatient and outpatient care for cellular, gene and immune cell-engaging therapies
Clinicians highlight the need to educate patients about warning signs
Immune toxicity remains a diagnosis of exclusion, and multidisciplinary collaboration remains the cornerstone for early diagnosis and treatment.
Benefits of neoadjuvant immunotherapy reflect emerging standard of care
Phase 2 trials investigate sitagliptin and methimazole as adjuvant therapies