Algorithm reveals many features not appreciated by the unaided eye
By Pallavi Tiwari, PhD; Volodymyr Statsevych, MD; Manmeet Ahluwalia, MD; and Stephen E. Jones, MD, PhD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
For over a century, malignant brain tumors such as glioblastoma (GBM) have carried a dismal prognosis. The most recent substantial advance has been provided by surgical resection and chemoradiation followed by adjuvant temozolomide therapy. Yet a problem during the requisite post-treatment surveillance imaging is that the brain’s reaction to heavy doses of radiation can mimic the appearance of true tumor progression on MRI (Figure 1).
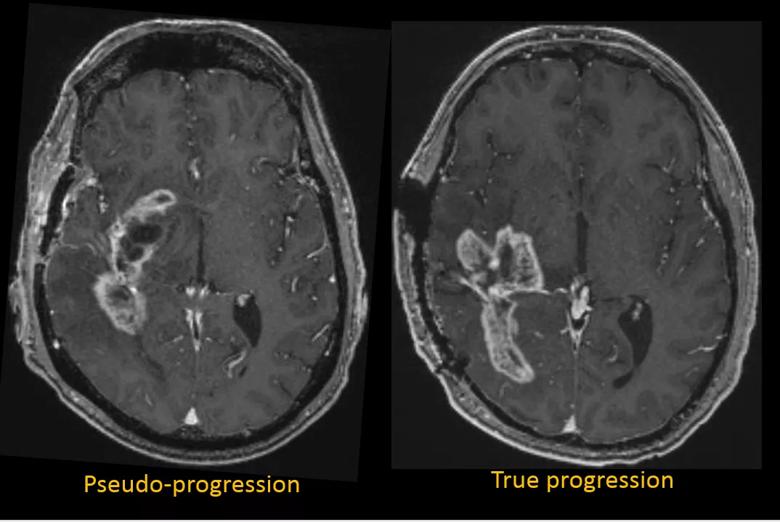
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/c09ca98d-910c-4764-96a5-1c26b3774718/19-NEU-5569-Inset1-glioblastoma-pseudoprogression_png)
Figure 1. The vexing similarities of glioblastoma pseudoprogression and true progression. Left: Axial T1-weighted MRI with gadolinium enhancement from a 56-year-old man with a right temporal glioblastoma after resection and radiation therapy. Comparison with prior studies (not shown) showed progression, but subsequent studies were unchanged in the context of a stable clinical picture. Overall the patient’s images were classified as pseudoprogression by the clinical team. Right: Axial T1-weighted MRI from a 44-year-old man with a right parietotemporal glioblastoma. Prior and subsequent studies showed continued progression. Together with the clinical picture, the patient’s images were classified as true progression by the clinical team.
Such “pseudoprogression” is not infrequent, occurring in up to about one-third of treated GBM cases, and therefore confounds the goal of response assessment and survival prediction. For example, if a new MRI shows an increase in the size of enhancing tissue, it becomes clinically challenging to decide between initiating a new treatment or continuing surveillance.
Advertisement
While capabilities have been enhanced by some advanced imaging techniques, such as MR perfusion, MR spectroscopy, MR permeability and PET radiotracers, these techniques often have limited applicability in clinical settings. Confirmed diagnosis comes solely from surgical biopsy or resection. Unfortunately, invasive biopsy carries risks of morbidity and mortality in addition to considerable financial burden, with each surgical intervention costing approximately $30,000 to $50,000. Moreover, patients with tumor progression have to wait for signs on additional scans to warrant an invasive biopsy for disease confirmation, which delays tumor-specific therapy. Therefore, there is great utility to be gained from any new noninvasive methodology to help discriminate tumor progression from pseudoprogression.
Our recent research takes a step back to use conventional MRI, but we have applied new machine learning techniques to assess for subtle distinguishing features not normally appreciated by the unaided eye. Such features, termed radiomics, can include details of lesion shape, relation of enhancing margins to adjacent edematous margins, eccentricity of the lesion, texture of the unenhanced parts of the tumor and texture of the enhancement.
One advantage of computerized assessment over the human eye is that it can be inherently three-dimensional, whereas visual perception begins with a two-dimensional assessment. This artificial intelligence approach is only now feasible thanks to the application of machine learning techniques, wherein an algorithm can be trained by “viewing” a large number of examples in a given category and is then able to “learn” any discriminating features on its own. The process is akin to how an infant learns to distinguish between cats and dogs — i.e., by seeing many examples of each while a parent says “dog” or “cat” as appropriate. While a child may take days to learn the difference, the algorithm can learn in seconds.
Advertisement
Another advantage of these approaches is that they allow us to explore the entire tumor environment (enhancing tumor as well as nonenhancing tumor/edema) instead of just a small fraction of tissue obtained via biopsy, which may provide incorrect results depending on the location from which it was taken.
Support for this approach is provided by a recent two-site study by our group.1 For the specific problem of identifying features of GBM progression versus pseudoprogression, we developed a machine learning algorithm using 38 examples of true GBM progression and 21 examples of true pseudoprogression. For each patient, the algorithm extracted the three-dimensional surface of both the enhancing tissue and the nonenhancing surrounding edema, and then computed measures for each surface, including curvedness, sharpness, shape index and total curvature (see examples in Figures 2 and 3). Positive features for radiation necrosis were found to be smooth elliptical shape and centricity, whereas positive features for progression were eccentricity and irregular shape. A hypothesized cause for such shape differences is uneven tumor growth due to irregular and aggressive tumor infiltration, likely influenced by underlying white matter structure.
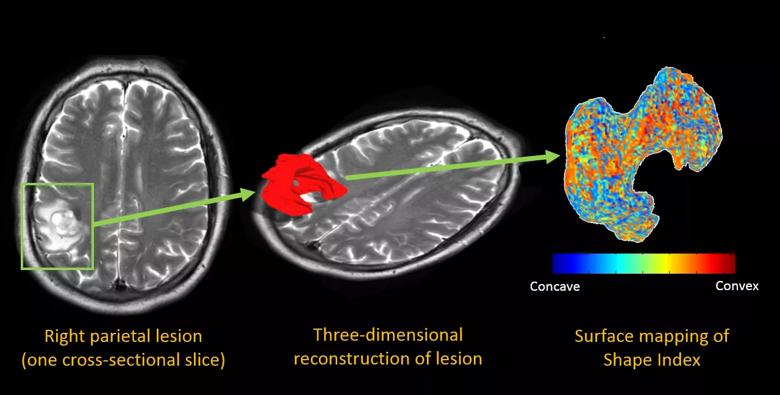
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/f5b8b5c1-6d44-47bc-8d2a-a102c8fb3759/19-NEU-5569-Inset2-parietal-edema-color-maps_png)
Figure 2. Color maps from a 59-year-old man with right parietal edema. The left panel shows a single axial T2-weighted image after radiation treatment, with imaging features ambiguous for radiation necrosis versus recurrent tumor. The middle panel overlays an extracted three-dimensional surface of the lesion, and the right panel shows the same surface with a colored overlay that maps the “shape index,” a measure of the concavity versus the convexity of the surface.
Advertisement
After this training of the machine learning algorithm was completed, the algorithm was tested on a new set of 46 cases — 33 with true GBM progression and 13 with true pseudoprogression — with the goal of confirming identification of statistically significant radiomic features distinguishing the two states. The algorithm was able to correctly distinguish true progression from pseudoprogression with an accuracy of 90.2%.1 These results are very promising, given that a similar study2 found expert radiologists’ accuracy in detecting radiation necrosis and tumor recurrence to be approximately 50%. Even biopsies are only 85% to 90% accurate, owing to challenges with tumor heterogeneity that may lead to unreliable diagnosis.
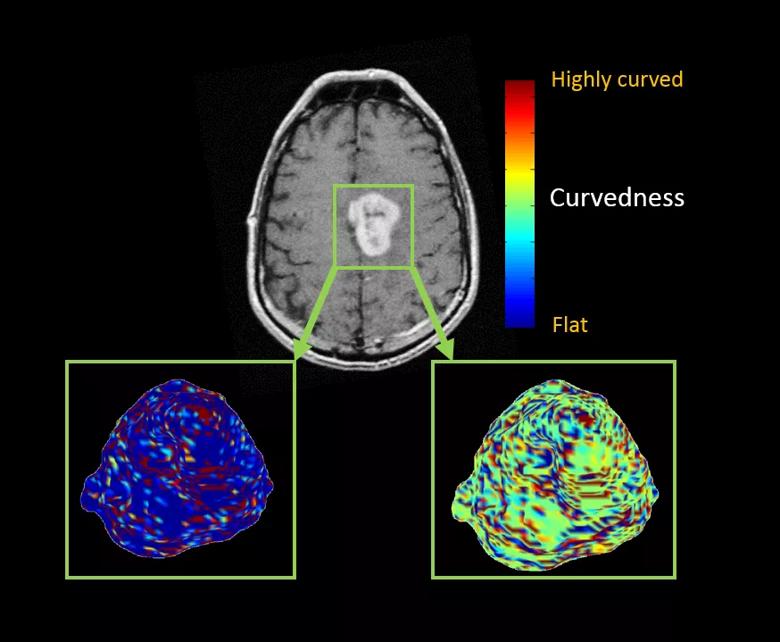
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/35412b31-6a8e-43cd-84cd-deed6ce0f84f/19-NEU-5569-Inset3-frontal-enhancing-lesion-color-maps_png)
Figure 3. Color maps from a 60-year-old woman who had a left medial frontal enhancing lesion. The top image shows a single axial T1-weighted post-contrast image demonstrating a solid avidly enhancing mass. Below are two maps showing the extracted three-dimensional surface of the mass. The left panel shows the sharpness measure, where areas with sharper surface curvature (higher) are in red, whereas flat areas with lower curvature values are in blue. The right panel shows the curvedness measures, where highly curved regions are in red and flat areas are in blue.
The inexorable advance of computing speed and memory is now crossing a threshold, allowing machine learning algorithms and other forms of artificial intelligence to make their first substantial contributions to some aspects of medical care. Our research demonstrates the successful application of such tools to extend interpretation of conventional imaging beyond the physician’s eye through the ability to measure, for example, subtle texture features or the details of convoluted three-dimensional shapes. Naturally, these algorithms are limited by the training they are provided, and human judgment is still required to assess for confounders such as artifacts.
Advertisement
Beyond treatment assessment, artificial intelligence is also finding applicability in predicting patient prognosis and treatment response in GBM.3,4 These applications are being developed with the aim of moving the needle toward more-personalized treatment decisions in GBM in the future. While artificial intelligence is unlikely to replace the human physician anytime soon, it will likely become an increasingly powerful tool in the physician’s arsenal.
Dr. Statsevych (statsev@ccf.org) is a staff physician in Cleveland Clinic’s Department of Diagnostic Radiology.
Dr. Jones (joness19@ccf.org) is a staff neuroradiologist and Vice Chairman for Research and Academic Affairs in Cleveland Clinic’s Imaging Institute.
Advertisement
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches