Brief profile of three tools that may help avoid the need for biopsy
By Mazen Hanna, MD, and Joseph P. Donnelly, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Diagnosis of cardiac amyloidosis (CA) starts with visualization of the two-dimensional (2-D) echocardiogram in conjunction with the electrocardiogram (ECG). The classic hallmark of CA is the combination of low voltage on ECG and increased left ventricular (LV) wall thickness on echocardiogram. Subsequent laboratory tests, cardiac imaging or tissue biopsy is used to confirm the diagnosis.
Over the past decade, the ability to diagnose CA noninvasively has dramatically improved with strain imaging using 2-D speckle tracking echocardiography, cardiac magnetic resonance imaging (MRI) and nuclear bone scintigraphy. These diagnostic tools have given clinicians options to pursue the diagnosis of CA without directly proceeding to endomyocardial biopsy.
We recently profiled these advanced noninvasive tools in a comprehensive review of CA management (“Cardiac amyloidosis: An update on diagnosis and treatment”) published in the Cleveland Clinic Journal of Medicine (2017;84[suppl 3]:12-26). To advance understanding of these noninvasive tools’ role in CA diagnosis and management, this post excerpts that discussion from the larger review article.
Longitudinal strain imaging measures the actual deformation of myocardium in specific LV segments, and quantification is displayed as a polar map, with a more negative value coded in red and associated with better function.
Our group, among others, has described a specific pattern in CA called “apical sparing,” in which the apical LV segments have normal or near-normal strain compared with the mid and basal segments. The easily recognizable bull’s-eye pattern on polar map can help differentiate CA from other forms of LV hypertrophy, such has hypertension or hypertrophic cardiomyopathy, with good sensitivity and specificity (Figure, panel A).1
Advertisement
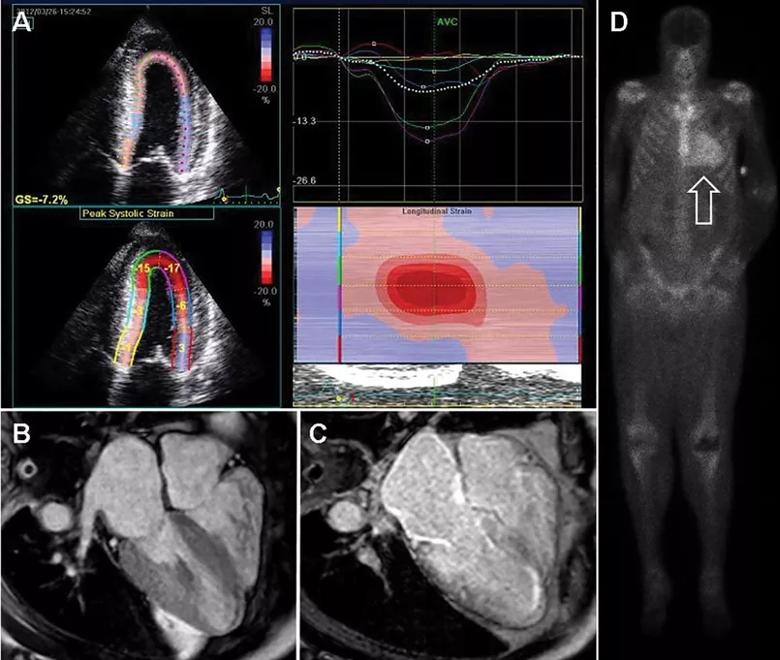
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5547f2c8-7b9d-45db-8842-3a59bdfd8164/18-HRT-412-Amyloidosis-CQD-Inset_jpg)
Figure. Noninvasive imaging for cardiac amyloidosis. (A) Longitudinal strain imaging using two-dimensional speckle tracking echocardiography reveals the characteristic bull’s-eye pattern of apical sparing. (B) Cardiac magnetic resonance imaging displays left and right ventricular thickening and, with contrast (C), a diffuse late gadolinium enhancement pattern that is diffuse and subendocardial, which also involves the right ventricle and left atrium. (D) 99mTechnetium pyrophosphate scan shows grade 3 myocardial radiotracer uptake characteristic of transthyretin cardiac amyloidosis. Reprinted, with permission, from Cleveland Clinic Journal of Medicine (2017;84[suppl 3]:12-26).
Cardiac MRI is useful for the diagnosis of CA (Figure, panels B and C). Imaging after administration of gadolinium contrast shows a characteristic late gadolinium enhancement (LGE) pattern that is diffuse and subendocardial and does not follow any particular coronary distribution.2 LGE can also be seen in the right ventricle and the atrial walls, and can be transmural and patchy in the acquired wild-type variant of transthyretin amyloidosis (ATTRwt). Sensitivity of cardiac MRI for the diagnosis of CA is about 90 percent, and specificity is about 70 to 80 percent, with an overall negative predictive accuracy of approximately 85 percent.3
One of the main limitations of cardiac MRI for the diagnosis of CA is the inability to give contrast in patients with reduced glomerular filtration rate. However, native T1-myocardial mapping techniques that do not require contrast show significantly increased native T1 times in CA and offer a promising alternative. Cardiac MRI parameters such as LGE, the difference in inversion time between the LV cavity and myocardium, native T1 mapping, and extracellular volume offer prognostic information. A greater than fivefold mortality increase is seen in CA patients with transmural LGE compared with those without LGE.2,3
Advertisement
99mTechnetium pyrophosphate (99mTcPYP), a radiotracer used in bone scans, was initially used in cardiology to quantify myocardial infarction due to its ability to localize calcium.4 Its potential utility in CA came in 1982 when diffuse myocardial 99mTcPYP uptake on cardiac radionucleotide imaging was noted in 10 patients with tissue-proven amyloidosis.5 Several subsequent studies reproduced and expanded upon this observation and revealed its diagnostic value, specifically showing that there is significant uptake in the transthyretin amyloidosis (ATTR) form of CA but mild to no uptake in the immunoglobulin light chain amyloidosis (AL) form of CA. This offers a significant advantage over other noninvasive modalities in that it not only confirms the diagnosis of CA but differentiates ATTR.6
99mTcPYP myocardial radiotracer uptake is graded by the semiquantitative visual score of cardiac retention, where 0 = no cardiac uptake, 1 = mild uptake less than bone, 2 = moderate uptake equal to bone and 3 = high uptake greater than bone (Figure, panel D).7 Additionally, quantitative analysis of heart retention can be calculated drawing circular regions of interest over the heart and mirrored on the contralateral chest wall. A heart-to-contralateral ratio greater than 1.5 is consistent with the diagnosis of ATTR.8,9
In 2016, a multicenter study showed that grade 2 or 3 myocardial radiotracer uptake on bone scintigraphy in the absence of evidence of a monoclonal gammopathy was diagnostic for ATTR, suggesting that this technique can be a cost-effective noninvasive modality in this setting.7
Advertisement
The full version of the Cleveland Clinic Journal of Medicine review article from which this is excerpted is freely available here.
Advertisement
Dr. Hanna is a cardiologist in the Section of Heart Failure and Cardiac Transplantation Medicine and Co-Director of Cleveland Clinic’s Amyloid Program. Dr. Donnelly is a research fellow in the Department of Cardiovascular Medicine.
Advertisement

Scenarios where experience-based management nuance can matter most

Introducing Krishna Aragam, MD, head of new integrated clinical and research programs in cardiovascular genomics

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients