Should we focus on the sugar or the weight?

By Bartolome Burguera, MD, PhD, Khawla F. Ali, MD, and Juan P. Brito, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Obesity is a leading public health concern, affecting nearly 60 million adult Americans.1 It is a major risk factor for the development of insulin resistance and type 2 diabetes mellitus (DM).2 More than 90% of patients with type 2 DM have obesity, and obesity is a major obstacle to achieving long-term glycemic control.3
Clinical studies have demonstrated that a 6- to 7-kg increase in body weight increases the risk of developing type 2 DM by 50%, while a 5-kg loss reduces the risk by a similar amount.4As a result, most patients who have a body mass index greater than 40 kg/m2 suffer from type 2 DM.5 Strong evidence exists that bariatric surgery and its resulting weight loss has positive effects on fasting blood sugar, hemoglobin A1c (HbA1c), lipid profiles, and other metabolic variables.6
When combined, obesity and type 2 DM carry a significant burden of micro- and macrovascular complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease. As a result, a high prevalence of morbidity and mortality is seen among patients with obesity and type 2 DM; those between the ages of 51 and 61 have a 7-times higher mortality rate compared with nonobese normoglycemic people, and patients with diabetes alone have a 2.6-times higher mortality rate.7
Although type 2 DM and obesity go hand in hand, clinicians tend to focus on the sugar and neglect the weight, concentrating their efforts on improving blood glucose indices, and prescribing in many instances medications that cause weight gain. As a result, we are faced with a rising epidemic of obesity, perpetuating a preexisting epidemic of diabetes.
Advertisement
An optimal, comprehensive approach to managing patients with type 2 DM should encompass both the control of dysglycemia and its associated comorbidities, obesity being the key player.8 However, clinical practice is often misaligned with the evidence. For instance, many of our first-line oral treatments for type 2 DM (except for metformin) are associated with weight gain.9 With time, control of glycemia becomes more and more ineffective, at which point therapy is intensified with insulin, further exacerbating the weight gain.10
Therefore, it seems counterintuitive to treat a disease for which obesity is one of the main risk factors with medications that promote weight gain. Yet healthcare providers are faced with a therapeutic dilemma: should they focus their efforts on improving patients’ glycemic control, or should they invest in helping these patients lose weight? Although an ideal approach would incorporate both aspects, the reality is that it is far from practical.
A few issues impinge on integrating weight loss in the care of type 2 DM. Although the American Medical Association recognized obesity as a disease in 2013,11 some providers still perceive obesity as a self-inflicted condition that is due to bad lifestyle and behavior.11 Many clinicians may also have low expectations for patients’ success, and often lack the time and knowledge to intervene regarding nutrition, physical activity, and psychological issues pertinent to the management of obesity in type 2 DM. Therefore, in many cases, it seems less complicated and more rewarding for both patients and physicians to concentrate on improving the HbA1c value rather than investing efforts in weight loss. For diabetic patients with obesity, this could mean that clinicians may prescribe glucose-lowering therapies, such as insulin and sulfonylureas, at the expense of weight gain. Additionally, clinicians often experience the need to provide recommendations more aligned with metrics that dictate reimbursement (eg, HbA1c targets) within healthcare systems that still raise concerns regarding obesity visit reimbursements.
Advertisement
Lastly, the lack of trustworthy or pertinent evidence (lack of comparative effectiveness research) for antiobesity medications may limit their use in daily practice. Physicians have had little confidence in the efficacy of antiobesity drugs, and often raise significant safety concerns, especially after witnessing important fiascos in this field, eg, dexfenfluramine, rimonabant, and sibutramine.2,12,13
As a result, many of our patients with obesity and type 2 DM may not consider the need for weight loss, and may not even be aware that type 2 DM is caused by obesity and physical inactivity in the first place. Others have accumulated a significant degree of frustration, and have “thrown in the towel” already after unsuccessful weight-loss efforts, many of which were not medically supervised.
For all of the above reasons, both clinicians and patients often concentrate their efforts on treating blood glucose numbers rather than the “obesity-diabetes” as a whole.14 And as a result, our practices are slowly filling up with patients with obesity and type 2 DM who are treated primarily with insulin, resulting in a progressive (and untreated) obesity and diabetes epidemic.
Because the body strongly defends its fat cells, the common advice to simply “eat less, move more” cannot be expected to bring about meaningful and lasting weight reduction or control of HbA1c. However, weight-loss drugs (Table 1),15 used in conjunction with an interdisciplinary lifestyle intervention program, may provide more success regarding both issues. Here we discuss a few pharmacologic therapies approved for the management of obesity in the context of type 2 DM, and vice versa. Taking into account that dosages of these medications should be individualized to achieve a weight-loss goal with the lowest effective dose possible.
Advertisement
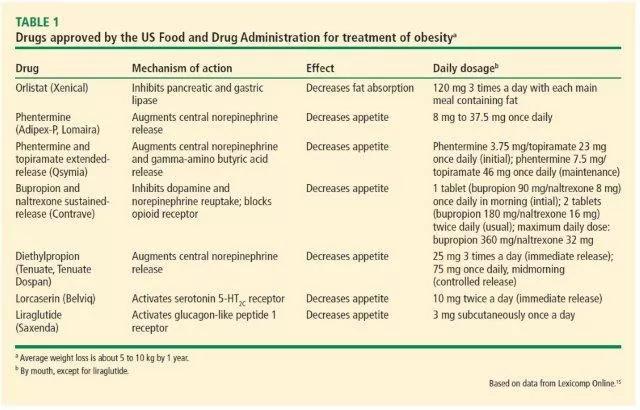
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/5dce026b-f9a6-4809-81d0-f493929b858f/table2_jpg)
Orlistat (Xenical) is the only weight-loss drug approved by the US Food and Drug Administration (FDA) that acts outside the brain. It inhibits pancreatic lipases, resulting in up to 30% less fat absorption in the gut. Orlistat has been approved for long-term use by the FDA.
Benefits. In the XENical in the Prevention of Diabetes in Obese Subjects study, treatment with orlistat resulted in a significant reduction in the cumulative incidence of type 2 DM after 4 years of treatment (9.0% with placebo vs 6.2% with orlistat), corresponding to a risk reduction of 37.3%.16Mean weight loss after 4 years was significantly greater in the orlistat group (5.8 vs 3.0 kg with placebo; P < .001).16 Other benefits of orlistat included a reduction in low-density lipoprotein cholesterol independent of that expected from change in body weight.16
Adverse effects include flatulence with discharge and fecal urgency after high-fat dietary indiscretions. Serum levels of fat-soluble vitamins (A, D, E, and K) were lower with orlistat than with placebo,16 and a fat-soluble vitamin supplement should be taken 2 hours before or after taking orlistat. Serious but very uncommon adverse events such as kidney damage have been reported.17 Kidney and liver function should be monitored while taking orlistat.
Phentermine (Adipex-P, Lomaira), a sympathomimetic amine, is the most commonly prescribed antiobesity drug in the United States. A schedule IV controlled substance, it is FDA-approved for short-term use (up to 12 weeks). Its primary mechanism of action is mediated by reduction in hunger perception. It was first developed in the 1970s and is available in doses ranging from 8 mg to 37.5 mg daily.18
Advertisement
Benefits. In a randomized trial, at 28 weeks, weight loss was 1.5 kg with placebo and 5.3 kg with phentermine.19 No long-term (> 1 year) randomized controlled trials of the effectiveness of phentermine monotherapy in weight loss have been conducted.
Adverse effects. Dizziness, dry mouth, insomnia, constipation, and increase in heart rate were most common.19
Phentermine is contraindicated in patients with coronary artery disease, congestive heart failure, stroke, and uncontrolled hypertension. Currently, no data exist on the long-term cardiovascular effects of phentermine. We believe phentermine, used in patients at low to intermediate cardiovascular risk, is a useful “jumpstart” tool, in combination with lifestyle changes, to achieve weight loss and improve metabolic values for those with type 2 DM and obesity.
Phentermine is a controlled substance per Ohio law. Patients must be seen once a month by the prescribing provider and prescriptions are limited to a 30-day supply, which must be filled within 7 days of the date of the prescription. Phentermine can only be prescribed for a maximum of 3 months and must be discontinued for 6 months before patients are eligible for a new prescription.
Obesity is a product of complex interactions between several neurohormonal pathways. Approaches simultaneously targeting more than one regulatory pathway have become popular and quite efficient strategies in treating patients with obesity.20 Stemming from such approaches, antiobesity drug combinations such as phentermine and topiramate extended-release (Qsymia) have become increasingly recognized and used in clinical practice. The combination of these 2 medications has been approved for long-term use by the FDA.
Phentermine and topiramate extended-release is a fixed-dose combination that was approved for weight loss in 2012. Topiramate, an anticonvulsant, and phentermine exert their anorexigenic effects through regulating various brain neurotransmitters and result in more weight loss when used together than when either is used alone. Several clinical trials evaluated the efficacy of low doses of this combination in weight loss.
Benefits. In a randomized trial in patients with obesity and cardiometabolic diseases, at 56 weeks, the mean weight loss was:
Patients in the active treatment groups also had significant improvements in cardiovascular and metabolic risk factors such as waist circumference, systolic blood pressure, and total cholesterol/high-density lipoprotein cholesterol ratio. At 56 weeks, patients with diabetes and prediabetes taking this preparation had greater reductions in HbA1c values, and fewer prediabetes patients progressed to type 2 DM.21
Adverse effects most commonly seen were dry mouth, paresthesia, and constipation.21
This combination is contraindicated in pregnancy, patients with recent stroke, uncontrolled hypertension, coronary artery disease, glaucoma, hyperthyroidism, or in patients taking monoamine oxidase inhibitors. Women of childbearing age should be tested for pregnancy before starting therapy, and monthly thereafter, and also be advised to use effective methods of contraception while taking the medication. Topiramate has been associated with the development of renal stones and thus should be used with caution in patients with a history of kidney stones.
Bupropion and naltrexone sustained-release (Contrave) is another FDA-approved combination drug for chronic weight management. Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for depression and smoking cessation, and naltrexone is an opioid receptor antagonist approved for treating alcohol and opioid dependence. The combination of these 2 medications has been approved for long-term use by the FDA.
Benefits. In a randomized trial in patients with obesity and type 2 DM, weight loss at 56 weeks was:
Absolute reductions in HbA1c were:
Improvements were also seen in other cardiometabolic risk factors such as triglyceride and high-density lipoprotein cholesterol levels.22
Adverse effects. The most common adverse effect leading to drug discontinuation was nausea. Other adverse effects reported were constipation, headache, vomiting, and dizziness.22
Naltrexone-bupropion is contraindicated in patients with a history of seizure disorder or a diagnosis of anorexia nervosa or bulimia, or who are on chronic opioid therapy.
Diethylpropion (Tenuate, Tenuate Dospan) is a central nervous system stimulant similar to bupropion in its structure. It was approved by the FDA for treating obesity in 1959. It should be used as part of a short-term weight-loss plan, along with a low-calorie diet. Diethylpropion is also a controlled substance and, as with phentermine therapy, patients are required to be seen once a month by their prescriber. Diethylpropion cannot be prescribed for more than 3 months.
Benefits. Weight loss in a randomized trial at 6 months:
After 6 months, all participants received diethylpropion in an open-label extension for an additional 6 months. At 12 months, the mean weight loss produced by diethylpropion was 10.6%.23 No differences in heart rate, blood pressure, electrocardiographic results, or psychiatric evaluations were observed.
Adverse effects. As with phentermine, common side effects of diethylpropion include insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, and palpitations.23
Lorcaserin (Belviq) was approved by the FDA for chronic weight management in June 2012. It exerts its effects through binding selectively to central 5-HT2C serotonin receptors, with poor affinity for 5-HT2A and 5-HT2B receptors. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn from the market in 1997 after being reported to be associated with valvular heart abnormalities.24 Lorcaserin has been approved for long-term use by the FDA.
Benefits. Mean weight loss at 1 year in the Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus trial25 was:
Absolute reductions in HbA1c values were:
Absolute reductions in fasting plasma glucose values were:
Adverse effects. The most common adverse effects were headache, dizziness, and fatigue. There was no significant increase in valvulopathy on echocardiography of participants receiving lorcaserin compared with placebo.25
Liraglutide (Saxenda, Victoza) is a glucagon-like peptide-1 (GLP-1) receptor agonist. Native GLP-1 is a hormone secreted by intestinal L cells in response to consumption of fat and carbohydrate-rich foods. It stimulates the release of insulin and suppresses any inappropriately elevated postprandial glucagon levels. In addition to its effect on glucose metabolism, GLP-1 also reduces appetite and delays gastric emptying in humans.26 Unlike the extremely short half-life of native GLP-1 (estimated at 1 to 2 minutes), liraglutide has a half-life of 13 hours, allowing it to be given once daily.26Liraglutide medication has been approved for long-term use by the FDA.
Benefits. The Liraglutide Effect and Action in Diabetes 1–5 studies compared the effects of liraglutide monotherapy with antidiabetic oral medications or insulin, as well as in combination with antidiabetic oral agents. Liraglutide (Victoza) at doses approved for type 2 DM of 1.2 mg and 1.8 mg daily had significant effects in reducing HbA1c by 0.48% to 1.84% and weight by 2.5 kg to 4 kg.27,28 At a dose of 3.0 mg, liraglutide (Saxenda) is approved for chronic weight management. This dose of liraglutide has been shown to be effective and safe in patients with type 2 DM and obesity.
In the 56-week SCALE Diabetes trial,29 liraglutide at a dose of 3.0 mg resulted in 6.0% weight reduction, compared with 2.0% in the placebo group. Of participants receiving 3.0 mg of liraglutide, 54.3% achieved more than 5% weight loss at 56 weeks compared with 21.4% with placebo. Liraglutide also resulted in significant improvements in HbA1c (mean change −1.3% vs −0.3% with placebo), fasting and postprandial glucose levels, and fasting glucagon levels.29
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial has shown liraglutide to significantly reduce rates of major cardiovascular events (first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in patients with elevated cardiovascular risk factors.30 These findings make liraglutide a favorable choice for high-risk patients with type 2 DM, obesity, and cardiovascular disease.
It is important to indicate that if a 5% weight loss is not achieved by 3 months with any of these weight-loss medications, it would be reasonable to stop the medication and consider switching to a different medication. These medications work best when combined with diet and increased physical activity. Weight-loss medications should never be used during pregnancy.
Women of childbearing age should be advised to use effective contraception methods while taking any of the above antiobesity medications.
Although not FDA-approved for weight management, metformin has anorexigenic effects that aid in weight loss. It also inhibits hepatic glucose production and improves peripheral insulin sensitivity, making it a useful agent in patients with type 2 DM and obesity.
A meta-analysis of 31 trials showed that metformin reduced body mass index by 5.3% compared with placebo.31Metformin should be considered as a first-line agent in obese patients with type 2 DM.
In healthy people, nearly all glucose is filtered in the glomerulus, but then 98% of it is reabsorbed in the proximal tubule by sodium-glucose cotransporter-2 (SGLT-2). Drugs that inhibit SGLT-2 increase urinary glucose excretion and, as a result, help control hyperglycemia. Another, off-label effect of excreting more glucose is weight loss: a sustained weight loss of about 3 kg to 5 kg in clinical studies.32 Although they can be used as monotherapies, SGLT-2 inhibitors are usually used as add-on therapies in patients with type 2 DM.
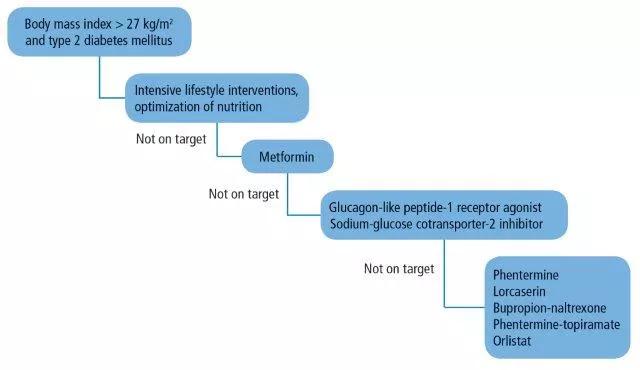
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/494e8361-a5c3-4e20-82aa-a692fe7aa501/burguera_antiobesitydrugsinmanagementofdiabetes_f1-2_jpg)
Figure 1. Therapeutic algorithm for patients with obesity and type 2 diabetes mellitus.
In an ever-changing field of antiobesity medicines, practitioners are challenged daily with the “when’s and how’s” of prescribing antiobesity drugs. The addition of type 2 DM to the picture makes the choice of drug therapy even more challenging. Here, we propose a practical therapeutic algorithm (Figure 1) that incorporates antiobesity drugs in the management of patients with type 2 DM and obesity.
First, we believe that lifestyle interventions by optimization of nutrition and physical activity should be the cornerstone therapy in the management plan of any patient with type 2 DM and obesity. These interventions are best implemented through a comprehensive, multidisciplinary approach that integrates the care of dietitians, physical therapists, exercise physiologists, psychologists, and social workers.33 Patients need also to be seen frequently, ie, at least once every 3 months. The possibility of seeing patients in group-shared medical appointments on a monthly basis could also be considered.
We also believe that metformin should be added early in the course of treatment for its known benefits of improving insulin sensitivity and suppressing appetite. Target HbA1c goals and body weight in patients with type 2 diabetes and obesity should be tailored to the individual based on age, general health status, risk of hypoglycemia, capacity to do physical activity, and associated comorbidities. If no improvements are seen (HbA1c > 7% and < 3 % weight loss) despite lifestyle changes and the addition of metformin, the possibility of adding a GLP-1 receptor agonist or an SGLT-2 inhibitor as a second-line therapy should be considered. Both classes of medications aid in lowering HbA1c and promote further weight loss.
If no clinical progress is achieved at 3 months, the possibility of adding an FDA-approved weight-loss medication, as discussed above, should be strongly considered. Of note, this algorithm targets different endogenous pathways for weight loss and thus minimizes weight regain through compensatory mechanisms.
Patient-centered care has become a core quality measure in our healthcare systems and a key to our patients’ success. The decision to start an antiobesity drug should therefore reflect careful consideration of medical and personal patient issues, all of which are valued differently by patients.34
Individualized therapy is even more relevant among patients suffering from a significant burden of disease. About 80% of patients with diabetes live with at least 1 other medical condition,35 and each of these patients spends over 2 hours a day, on average, following doctors’ recommendations.36 If antiobesity medications are prescribed without careful consideration of the patient’s preexisting workload, they will be destined to fail. Therefore, it becomes crucial to first account for the patient’s ability to cope with therapy intensification. This requires careful deliberation between healthcare providers and patients, in aims of targeting a weight-loss plan that fits patients’ goals and is aligned with providers’ expectations.
Healthcare systems also play a key role in supporting better conversations about obesity in type 2 DM patients. They could implement multifaceted initiatives to promote shared decision-making and the use of decision aids to advance patient-centered obesity practices.37 Policymakers could redesign quality measures aimed at capturing the quality of obesity conversations, and develop policies that support better education for clinicians regarding the importance of addressing obesity with adequate communication and patient-centered skills. Guidelines are often too disease-specific and do not consider comorbidities in their context when providing recommendations.38 Thus, diabetes societies should respond to the need to guide care for patients with diabetes and its comorbidities, particularly obesity.
Obesity is a serious global health issue and a leading risk factor for type 2 DM. Lifestyle measures are the cornerstone of preventing and treating obesity and type 2 DM. Emerging data support the effectiveness of intensive, interdisciplinary weight-loss programs in patients with diabetes. The use of antiobesity drugs should be considered in patients who have not achieved adequate responses to lifestyle interventions. Medications should be tailored to the individual’s health risks and metabolic and psychobehavioral characteristics. In many cases, the addition of weight-loss drugs will help accomplish and maintain the recommended 10% weight reduction, resulting in improvement in glycemic control and significant reduction in cardiovascular risk factors. New studies combining antiobesity and antidiabetes medications in the context of lifestyle interventions will help define the optimal therapeutic approach for patients with type 2 DM and obesity.
This article originally appeared in the Cleveland Clinic Journal of Medicine (2017 July;84[suppl 1]:S39-S46).
Advertisement

Pheochromocytoma case underscores the value in considering atypical presentations

Advocacy group underscores need for multidisciplinary expertise

A reconcilable divorce

A review of the latest evidence about purported side effects

High-volume surgery center can make a difference

Advancements in equipment and technology drive the use of HCL therapy for pregnant women with T1D

Patients spent less time in the hospital and no tumors were missed

A new study shows that an AI-enabled bundled system of sensors and coaching reduced A1C with fewer medications