Incidence, outcomes and management
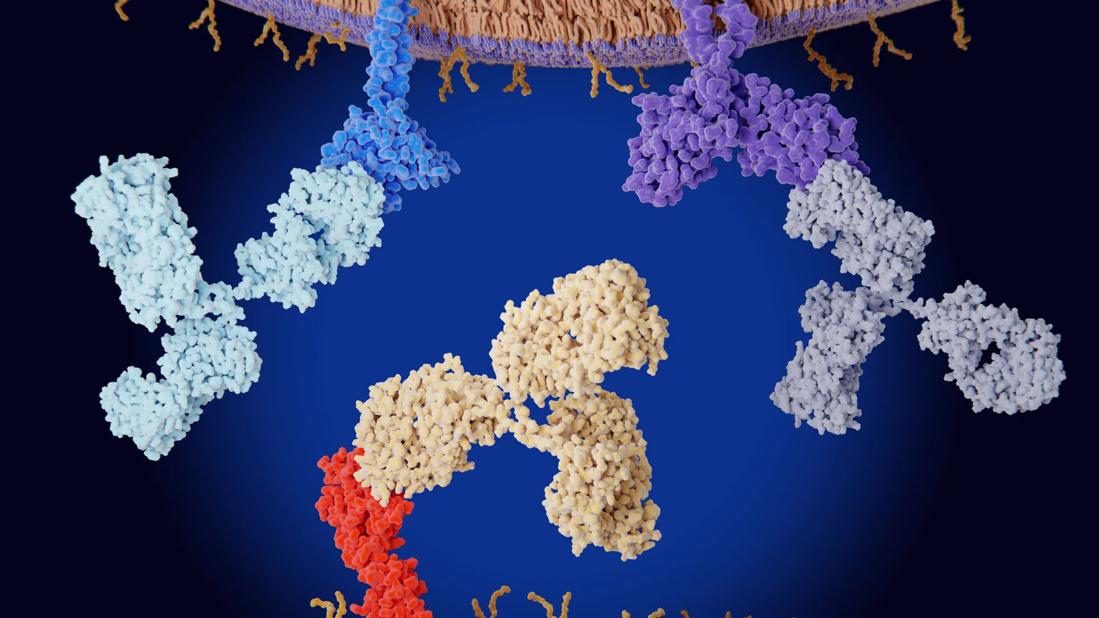
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/82d49c94-3333-4ed0-8474-3d8809fc969b/immune-1407321194)
Checkpoint inhibitors
This article is republished from the Cleveland Clinic Journal of Medicine, May 01, 2023; Volume 90, Issue 5
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
By Randol Kennedy, MD, FACP; Daniela Ciltea, MD; Hussein Awada, MD; Michael Morocco, MD; Naga Vura, MD
Immune checkpoint inhibitors are used more and more to treat several types of cancer, significantly extending cancer-free survival. However, concerns are growing about their toxic effects, which are many and varied.
Endocrinopathies are some of the most frequently reported adverse effects, and thyroid dysfunction is the most common of these. Here, we review the incidence and severity of each immune checkpoint inhibitor-related endocrinopathy, possible factors related to toxicity risk, and principles of management.
The U.S. Food and Drug Administration has so far approved nine immune checkpoint inhibitors, which variously target programmed cell death protein 1, programmed cell death ligand 1, cytotoxic T-lymphocyte–associated protein 4, and lymphocyte activation gene 3. Checkpoint inhibitor drugs have revolutionized cancer treatment, as they unleash the power of the immune system to destroy cancer cells.
Professional societies have issued guidelines for surveillance and treatment of immune checkpoint inhibitor-associated endocrinopathies. With time and further research, strategies for predicting, preventing and treating these toxicities should emerge.
The discovery of the molecular mechanisms by which cancer cells evade the immune system has brought about a revolution in cancer immunotherapy. In the past, immunotherapy had very limited success, but unmasking these mechanisms paved the way toward the invention of immune checkpoint inhibitors—monoclonal antibodies that block key regulators of the immune system. Cancer cells typically target these regulators, suppressing the immune response against them and thereby helping them evade the immune system.
Advertisement
Starting with ipilimumab in 2011, the FDA has so far approved nine immune checkpoint inhibitors that target the following proteins:
• Programmed cell death protein 1 (PD-1, less commonly known as CD279)
• Programmed cell death ligand 1 (PD-L1, also known as CD274)
• Cytotoxic T-lymphocyte–associated protein 4 (CTLA-4, also known as CD152)
• Lymphocyte activation gene 3 (LAG-3).
These drugs have become mainstays in treating a variety of tumors, including those of the lung, esophagus, stomach, colon, liver, kidney, bladder, uterus and skin.1 In fact, their efficacy has overtaken that of standard treatments, prolonging survival even in patients with tumors of advanced stage.
Nevertheless, concerns about the immune-related adverse effects of these drugs have been growing.2 The excessive activation of the immune system by these drugs causes dermatologic, endocrine, gastrointestinal, pulmonary, and other toxicities.2 In particular, endocrinopathies occur in roughly 10% of patients who receive immune checkpoint inhibitors.3 Hypopituitarism, type 1 diabetes mellitus, and thyroid and adrenocortical dysfunction are the most common disorders that checkpoint inhibitors cause, depending on the drug.4 The severity of these events has prompted researchers to look for adjuncts to minimize the toxicities while maintaining the efficacy of the drugs.3
Here, we review the mechanisms of action of the currently approved immune checkpoint inhibitors, the incidence of their associated endocrinopathies, the short-term and long-term outcomes of these adverse effects, and their management based on current guidelines.
Advertisement
The PD-1/PD-L1 pathway
PD-1, a cell-surface protein, was discovered by Ishida and colleagues5 while studying apoptosis. It is most notably expressed by activated cytotoxic T cells after recognizing non-self-antigens presented by major histocompatibility complexes of antigen-presenting cells.6 The interaction of the T-cell receptor and the major histocompatibility complex results in release of cytokines that trigger expression of PD-L1 by local parenchymal tissue.7 Parenchymal PD-L1 then binds
T-cell PD-1 to transmit an inhibitory signal to the T cell and induce peripheral immune tolerance, so that healthy parenchymal tissue is protected from inflammatory destruction.6,7
Tumor cells manipulate this pathway by overexpressing PD-L1, so that T cells become exhausted and apoptosis is inhibited.6 Therefore, blocking either PD-1 or PD-L1 enhances cytotoxic T-cell activity against PD-L1-expressing cells, including those of both the tumor and the parenchyma. As of today, four PD-1 inhibitors (nivolumab, pembrolizumab, cemiplimab and dostarlimab) and three PD-L1 inhibitors (atezolizumab, avelumab and durvalumab) have been approved by the FDA.4
The CTLA-4 pathway
CTLA-4 is another T-cell surface protein that transmits inhibitory signals when bound by its ligands.8 It is homologous to T-cell receptor, which, in contrast, transmits stimulatory signals when bound. CTLA-4 binds the T-cell receptor ligands CD80/86 on antigen-presenting cells with greater affinity and avidity than T-cell receptor.9 Thus, it can outcompete T-cell receptor for its ligands and prevent its downstream stimulatory signal. While this pathway is not manipulated by tumor cells, blocking CTLA-4 to ease T-cell receptor-CD80/86 binding enhances T-cell activation and cytotoxic activity against tumors.9 Ipilimumab remains the only FDA-approved CTLA-4 inhibitor to date.4
Advertisement
The LAG-3 pathway
The role of the LAG-3 pathway in tumorigenesis has been extensively studied since its discovery more than 30 years ago.10 LAG-3 is a transmembrane protein that binds major histocompatibility complex class II, suppressing proliferation and activation of T cells.10 This protein is also expressed on B cells and therefore has similar regulatory effects on B cells and natural killer cells.10 Naive T cells express low levels of LAG-3, but tumor antigens cause an increase in activity of LAG-3, leading to T-cell exhaustion.10
Inhibiting the LAG-3 pathway restores T-cell function, thereby leading to increased accumulation and effector function on tumor cells.10 Of note, combining LAG-3 inhibition with PD-1 blockade reduces tumor burden synergistically.11 In March 2022, the FDA approved the first human LAG-3 inhibitor (relatlimab), to be used in combination with nivolumab to treat unresectable or metastatic melanoma, based on data from a randomized phase 2 and 3 study.12
Pituitary dysfunction
Hypopituitarism is a rare endocrine disorder that can result from disease of the pituitary gland or the hypothalamus. Hypophysitis, i.e., inflammation of the pituitary gland, usually leads to pituitary enlargement 13,14 and has been reported to be a major cause of immune checkpoint inhibitor-mediated hypopituitarism, although some authors use the terms hypopituitarism and hypophysitis interchangeably.15
As the use of immune checkpoint inhibitors has increased in recent years, so has the incidence of hypophysitis.13,14 Immune checkpoint inhibitor-induced hypophysitis affects the anterior pituitary (which secretes follicle-stimulating hormone, luteinizing hormone, adrenocorticotropic hormone, thyroid-stimulating hormone, prolactin, endorphins and growth hormone) more often than it affects the posterior pituitary (which secretes antidiuretic hormone and oxytocin),16,17 and most patients have multiple hormonal deficiencies. Barroso-Sousa et al 3 reported in a meta-analysis that 36 (39%) of 92 patients on immune checkpoint inhibitor regimens who developed hypophysitis had symptoms of grade 3 or higher on the Common Terminology Criteria for Adverse Events (CTCAE) scale. 3,18
Advertisement
Central hypothyroidism is the most frequent complication, followed by hypogonadism. This is distinctive with the CTLA-4 inhibitor ipilimumab, suggesting that CTLA-4 is expressed preferentially by the thyrotropin-secreting and gonadotropin-secreting cells.16,19
Central adrenal insufficiency is also common and is concerning, as it can lead to life-threatening adrenal crisis. Hyperprolactinemia: Prolactin levels are usually low; hyperprolactinemia is uncommon.17 Growth hormone deficiency is rare, as the growth hormone axis is usually spared.14 Diabetes insipidus a very rare feature of hypopituitarism.20
Risk factors for pituitary dysfunction
Male sex seems to play a role in incidence, with higher rates reported in men.16,17,21 Although this male predominance may be confounded by the sex discrepancies associated with melanoma (which also occurs more frequently in men, and which is treated with ipilimumab), the rates of hypophysitis still appear to be higher after taking this into account.16,17 This is in contrast to other etiologies of autoimmune hypophysitis, which are more common in women.14 Age is a contributing factor, with people over age 65 having a higher risk.16,17
Ipilimumab. Immune checkpoint inhibitor-induced hypophysitis-hypopituitarism is almost exclusively associated with the CTLA-4 inhibitor ipilimumab, and it appears to be the most common endocrinopathy associated with this drug,14 with incidences in the range of 10% to 15% reported.16,17 Cumulative dosage or cycle frequency do not appear to affect the incidence significantly.16 However, the incidence is significantly higher with nivolumab-ipilimumab combination therapy (about 8%) than with ipilimumab alone (about 4%).3 Hypophysitis-hypopituitarism occurs significantly less often with PD-1 inhibitors than with ipilimumab, and the presentations may drastically differ between the two drug classes, strongly suggesting independent pathways.22
For instance, gland enlargement and combined axis dysfunction are more common in those treated with ipilimumab, whereas secondary adrenal insufficiency with subtle gland enlargement is more common with PD-1 inhibitors.22
Human leukocyte antigen (HLA)-DR15.
Due to the pathogenic nature of immune-related adverse events in general, predisposing HLA variants have been researched as a way to predict adverse outcomes. So far, studies have revealed an association between HLA-DR15 and the development of immune checkpoint inhibitor-induced secondary insufficiency.23
Course of hypopituitarism
The median time of onset of hypophysitis-hypopituitarism is eight to 10 weeks after initiating treatment,16,17 although this can vary by as much as four months.24 Unlike other forms of autoimmune hypophysitis, it is usually not accompanied by visual disturbances. Pituitary enlargement and hypophysitis usually resolve, but hypopituitarism can persist (with or without steroid treatment) and may be permanent depending on the hormonal axis involved.24 For example, the thyroid axis may recover in the long term, but recovery of corticotroph cell function is rare. Therefore, quality of life after immune checkpoint inhibitor therapy poses a major issue for patients with secondary adrenal insufficiency.
Thyroid dysfunction
Thyroid dysfunction is the most common endocrine immune-related adverse event associated with immune checkpoint inhibitor therapy. Dysfunction can be in the form of either thyrotoxicosis or hypothyroidism, but the latter is the more common presentation.13
Although some authors use the terms thyrotoxicosis and hyperthyroidism interchangeably, we would like to clarify the definitions. Hyperthyroidism is thyroid hormone overproduction caused by an intrinsic pathological excess in thyroid hormone synthesis and secretion by the thyroid gland, and examples are Graves disease, toxic adenoma, and toxic multinodular goiter. Thyrotoxicosis, however, encompasses all causes of thyroid hormone excess, including hyperthyroidism and pathologies that result in a temporary excess release of thyroid hormone, as we will describe later. Thyrotoxicosis as the primary presentation is predominantly related to silent or destructive thyroiditis, and in most of these cases, hypothyroidism ensues shortly thereafter (median time 42 days).14,25 Therefore, many thyrotoxic adverse events may go undetected without close monitoring.14
For example, in a study by Lee et al, 25 of 45 patients who developed thyroid dysfunction after anti-PD-1 monotherapy or combination therapy, thyrotoxicosis was the initial presentation in 78% of patients, although 80% of those patients subsequently developed hypothyroidism.25 A study by Lu et al26 showed that only 9.3% of hypothyroidism cases reported to the FDA reporting system manifested with destructive thyroiditis (initially presenting as hyperthyroidism). Graves disease is also an uncommon presentation of immune checkpoint inhibitor-induced thyrotoxicosis.13 However, due to inconsistency in reporting, the incidence of Graves disease as an adverse event may be underreported.13,27
Barroso-Sousa et al3, in a meta-analysis of 38 randomized controlled trials, calculated the overall incidence of hypothyroidism to be 6.6% (95% confidence interval 5.5%–7.8%) and the incidence of thyrotoxicosis to be 2.9% (95% confidence interval 2.4%–3.7%).3,14 Most recently, Lu et al,26 using data from the FDA Adverse Event Reporting System (FAERS) from 2011 until 2020, reported a much lower incidence of thyroid dysfunction— 2.6%. Of these cases, 62% were hypothyroidism, 22.7% were hyperthyroidism, and 15.3% were reported as thyroiditis without thyroid function information.26 The authors accounted for this discrepancy as simply due to differences in study design and reporting, as clinical trials typically have closer follow-up and therefore more frequent reporting. Also, the FAERS may not capture less-severe adverse events.
However, recent clinical data strongly suggest that the incidence of immune checkpoint inhibitor-induced thyroiditis and hypothyroidism is increasing,28,29 which is to be expected given the increased screening and reporting as well as increased use of immune checkpoint inhibitors.
Regarding severity, most of the thyroid-related adverse events are either asymptomatic (subclinical) or cause mild or moderate symptoms (CTCAE grade 1 or 2), with less than 1% leading to severe symptoms, hospitalization or death (CTCAE grade 3 or higher).30
Risk factors for thyroid dysfunction
Women may have a higher risk of thyroid dysfunction than men.15,26,31,32 An explanation may be related to sex-hormone-mediated immune regulation or possibly sex-specific autoimmunity.33
Elevated body mass indexmay also be associated with increased risk, earlier onset of symptoms, and overt hyperthyroidism.15,31
Advanced age may also increase the risk of severe thyroid dysfunction, leading to increased rates of hospitalization, morbidity and death.26
Tyrosine kinase inhibitor use may also predispose to immunotherapy-related thyroid dysfunction,30,34 although tyrosine kinase inhibitors can also independently cause thyroid dysfunction.35
Biomarkers that may predict thyroid-related adverse events are elevated levels of thyroid-stimulating hormone, thyroid autoantibodies, thyroglobulin and cytokines.15,31,36 In addition, Kurimoto et al 36 demonstrated that higher serum levels of interleukin 1-beta, interleukin 2 and granulocyte-macrophage colony-stimulating factor at baseline and early decreases in interleukin 8, granulocyte colony-stimulating factor, and monocyte chemoattractant protein 1 were significantly associated with thyroid dysfunction (P < .5).36
The incidence of thyroid dysfunction strongly depends on the type of agent and whether it is given as monotherapy or combined therapy. For instance, studies by Barroso-Sousa et al3 and Lu et al26 clearly demonstrated that the anti-PD-1 class poses the highest risk of thyroid dysfunction. On the other hand, the anti-CTLA-4 agent ipilimumab is associated with the lowest frequency of thyroid dysfunction. Combination anti-CTLA-4 and anti-PD-1 therapy arguably has the highest risk of thyroid dysfunction. Of note, whereas previous studies consistently reported a higher incidence of thyroid dysfunction with combination anti-CTLA-4–anti-PD-1 therapy than with monotherapy of each class,3,4
Lu et al recently reported that the incidence of PD-1-related hypothyroidism exceeded that with combination therapy.26 However, this pattern of more adverse events with combination therapy is not unique to thyroid dysfunction, as discussed above with pituitary dysfunction. Also, higher CTCAE grades of thyroid dysfunction are more frequent with combination therapy.3,13
Dose and tumor type are not significantly associated with the incidence of immunotherapy-mediated thyroid dysfunction.3,13
Course of thyroid dysfunction
The time of onset of thyroid dysfunction varies greatly, within the first 15 weeks of therapy in most reported cases,37 but as early as seven days or as late as three years in others.14 Also, the time to onset is shorter with combined immunotherapy than with monotherapy.25 Most cases of hypothyroidism remain permanent and require long-term levothyroxine replacement therapy.13,14
Pancreatic endocrine dysfunction, diabetes mellitus
The adverse effects of immune checkpoint inhibitor therapy on pancreatic endocrine function manifest in a similar manner to type 1 diabetes mellitus, with low or undetectable C-peptide and elevated autoantibody levels.14,3 Incidence rates have been reported to be between 0.2% and 0.9%, with 0.1% being CTCAE grade 3 or higher.3
However, there are recent reports of a more fulminant course with rapid-onset diabetic ketoacidosis39,40 associated with a disproportionately normal to mildly elevated hemoglobin A1c.38,41 Incidence rates are higher with PD-1 inhibitors (nivolumab, pembrolizumab), followed by PD-L1 inhibitors.38,41
Combination therapy may also increase risk, with a shorter onset of symptoms after initiation of therapy.38,41 Akturk et al,38 in a systematic review and meta-analysis of 71 cases, reported that the mean age of the patients was 61.7 (± 12 years), 55% of cases were in men, and the median time to onset was 49 days (range five to 448 days) after starting treatment. Half of the patients had autoantibodies at presentation, with a higher incidence of diabetic ketoacidosis and more rapid onset of diabetes mellitus than in patients without autoantibodies. An at-risk DR or DQ allele as present in 85% of patients tested, similar to the rate in childhood-onset diabetes.38
In a systematic review, de Filette et al41 reported comparable results, with similar incidences of diabetic ketoacidosis (71%) and islet autoantibodies (53%). However, fewer patients (65%) had susceptible HLA genotypes. These findings suggest a role of allele screening in patients who may be at risk of immune checkpoint inhibitor-induced diabetes. As in childhood-onset type 1 diabetes, lifelong insulin therapy is needed, and unlike other immune checkpoint inhibitor endocrinopathies, pancreatic dysfunction does not respond to immunosuppressive therapy.42
Adrenal gland dysfunction
Immune checkpoint inhibitor-associated primary adrenal insufficiency is an infrequent manifestation of immune-related adverse events, accounting for less than 2%. Barroso-Sousa et al,3 in their meta-analysis and systematic review, reported only 43 cases of any grade primary adrenal insufficiency among 5,831 patients (0.7%), of which 14 (0.3%) were grade 3 or higher.
In another study, among 256 patients who received ipilimumab, two cases of primary adrenal insufficiency (0.8%) were observed.43 Grouthier et al44 reported that, of a total of 50,108 immune checkpoint inhibitor-associated adverse events (reported using the World Health Organization’s pharmacovigilance database of individual case safety reports over a decade), there were 451 cases of primary adrenal insufficiency, of which 45 were “definite” and 406 “possible.” A small majority (51.8%) of the cases were in men, with a median age of 66 years. Most patients had received immune checkpoint inhibitor monotherapy (nivolumab 44.3%, pembrolizumab 11.7% and ipilimumab 23.6%), and 17.9% had received combination therapy. The median time to onset was 120 days (range six to 576).44
Immune checkpoint inhibitor-associated primary adrenal insufficiency is associated with significant rates of morbidity (> 90% of cases are severe) and mortality (7.3%). Mortality rates were similar in the subgroups receiving combination therapy versus monotherapy.44
Melanoma recurrence may also be a concern, due to persistently elevated adrenocorticotropic hormone and melanocyte-stimulating hormone levels, although no studies to date have fully investigated this theory.45
Other endocrinopathies
Primary hypoparathyroidism,46 lipodystrophy47 and polyendocrine syndrome48 have been reported in case reports, but further characteristics are yet to be determined.
The American Society of Clinical Oncology,49 the National Comprehensive Cancer Network50 and the Society of Immunotherapy of Cancer51 have published practice guidelines on the recognition and management of immune checkpoint inhibitor-related endocrinopathies. The guidelines have similarities, but those of the American Society of Clinical Oncology are the most comprehensive, addressing acute management by CTCAE grade as well as when to consider endocrinology referral for thyroid dysfunction, hypopituitarism, adrenal insufficiency, and diabetes.49
The American Association of Clinical Endocrinology52 has also published a clinical review on the evaluation and management of immune checkpoint inhibitor-mediated endocrinopathies, which shares a similar approach. However, it recommends a low threshold for endocrinology referral in the event of any laboratory derangement suggesting endocrine organ dysfunction.
This includes thyroid dysfunction, as closer monitoring and further tests for adrenal insufficiency may be warranted. Generally, unless patients have moderate or severe symptoms (CTCAE grade 3 or 4), immune checkpoint inhibitor therapy can continue throughout the endocrine adverse event. Permanently stopping these drugs is not routinely recommended,49 as most endocrinopathies are long-term and are treatable. This is unlike other immune-related adverse events (eg, pulmonary toxicities), for which more aggressive temporary or permanent discontinuation is recommended.49
Also, unlike in other organ-specific immune-related adverse events, steroids are not routinely
recommended except in hypophysitis and primary adrenal insufficiency. High-dose steroids do not improve recovery rates in patients with hypophysitis53 and have been associated with worse outcomes.54 Therefore, high-dose steroids are reserved for patients with associated mass-effect symptoms. Similar outcomes have also been shown in patients with immune checkpoint inhibitor-induced diabetes,42 and steroids are generally avoided.
Dehydroepiandrosterone replacement is controversial. However, deficiency can be tested and treated in women with low libido or energy who are judged to be otherwise well-replaced for other hormonal deficiencies. The adverse effects of immune checkpoint inhibitors do have an upside: they may predict that the drug is working on the cancer, and the patient can expect prolonged recurrence-free survival.55 In particular, endocrine immune-related adverse events (particularly thyroid dysfunction) may have the strongest associations with improved clinical outcomes.56 These correlations therefore further support the consideration of not discontinuing immune checkpoint inhibitor therapy once the adverse events are manageable.
With the increased use of immune checkpoint inhibitors in cancer treatment, more precise reporting of their extensive endocrine immune-related adverse events is occurring. This will give a clearer picture of the true incidences and characteristics of each endocrinopathy and will help inform further updated guidelines for pretreatment investigations, monitoring and management. Prolonged routine post-treatment monitoring should also be considered, as some adverse effects can occur many months after discontinuation.
Interspecialist planning and monitoring with endocrinologists and oncologists should also be considered, as this may allow earlier hormone-replacement therapy, as well as immunotherapy de-escalation for those with moderate to severe events. Primary care physicians should be aware of the need to perform routine screening and surveillance investigations, when to consider endocrinology referral, and when to consider urgent or emergent care referral.
Patients should be routinely and extensively counseled on the endocrine adverse effects, including the common signs and symptoms, when to seek urgent care, and the likelihood of indefi nite hormonal replacement therapy should these events occur. Finally, larger prospective studies are needed to answer questions pertaining to risk factors (age, sex, autoimmune markers), interventions to reduce risk of endocrinopathies, and the risk of recurrence after restarting therapy.
1. Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J 2021; 23(2):39.
2. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016; 27(4):559–574.
3. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018; 4(2):173–182.
4. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res 2019; 51(3):145–156.
5. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992; 11(11):3887–3895.
6. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations and clinical outcome. Front Pharmacol 2017; 8:561.
7. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14(8):561–584.
8. Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994; 1(5): 405–413.
9. Guo Q, Huang F, Goncalves C, Del Rincón SV, Miller WH Jr. Translation of cancer immunotherapy from the bench to the bedside. Adv Cancer Res 2019; 143:1–62.
10. Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 2007; 117(11):3383–3392
11. Goding SR, Wilson KA, Xie Y, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol 2013; 190(9):4899–4909.
12. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022; 386(1):24–34.
13. Stelmachowska-Banas M, Czajka-Oraniec I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: an updated review. Endocr Connect 2020; 9(10): R207–R228.
14. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev 2019; 40(1):17–65.
15. Anderson B, Morganstein DL. Endocrine toxicity of cancer immunotherapy: clinical challenges. Endocr Connect 2021; 10(3):R116–R124.
16. Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 2014; 99(11):4078–4085.
17. Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment and biologic insights. Pituitary 2016; 19(1):82–92.
18. US Department of Health and Human Services National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0, Published November 27, 2017.
19. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014; 6(230):230ra45.
20. Nallapaneni NN, Mourya R, Bhatt VR, Malhotra S, Ganti AK, Tendulkar KK. Ipilimumab-induced hypophysitis and uveitis in a patient with metastatic melanoma and a history of ipilimumab-induced skin rash. J Natl Compr Canc Netw 2014; 12(8):1077–1081.
21. Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 2016; 186(12):3225–3235.
22. Faje A, Reynolds K, Zubiri L, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol 2019; 181(3): 211–219.
23. Yano S, Ashida K, Sakamoto R, et al. Human leucocyte antigen DR15, a possible predictive marker for immune checkpoint inhibitor-induced secondary adrenal insufficiency. Eur J Cancer 2020; 130:198–203.
24. Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol 2018; 178(2):173–180.
25. Lee H, Hodi FS, Giobbie-Hurder A, et al. Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res 2017; 5(12):1133–1140.
26. Lu D, Yao J, Yuan G, Gao Y, Zhang J, Guo X. Immune checkpoint inhibitor-related new-onset thyroid dysfunction: a retrospective analysis using the US FDA adverse event reporting system. Oncologist 2022; 27(2):e126–e132.
27. Tan MH, Iyengar R, Mizokami-Stout K, et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin Diabetes Endocrinol 2019; 5:1.
28. Shariff AI, Qamar A, Velez Rivera J, et al. SAT-414 a single center retrospective analysis and review of endocrinopathies from immune checkpoint inhibitors between 2007 and 2017. J Endocr Soc 2020; 4(suppl 1):SAT–414.
29. Brody HM, Macherla S, Bulumulle A, Namireddy P, Cherry, CR. The real-world incidence of immunotherapy-related thyroid dysfunction: a retrospective analysis of a single center’s experience over five years. J Clin Onc 2020; 38(suppl 5):98.
30. Zhan L, Feng HF, Liu HQ, et al. Immune checkpoint inhibitors-related thyroid dysfunction: epidemiology, clinical presentation, possible pathogenesis and management. Front Endocrinol (Lausanne) 2021; 12:649863.
31. Pollack R, Ashash A, Cahn A, Rottenberg Y, Stern H, Dresner-Pollak R. Immune checkpoint inhibitor-induced thyroid dysfunction is associated with higher body mass index. J Clin Endocrinol Metab 2020; 105(10):dgaa458.
32. Zhai Y, Ye X, Hu F, et al. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging U.S. Food and Drug Administration adverse events reporting system. J Immunother Cancer 2019; 7(1):286.
33. Triggianese P, Novelli L, Galdiero MR, et al. Immune checkpoint inhibitors-induced autoimmunity: the impact of gender. Autoimmun Rev 2020; 19(8):102590.
34. Sbardella E, Tenuta M, Sirgiovanni G, et al. Thyroid disorders in programmed death 1 inhibitor-treated patients: is previous therapy with tyrosine kinase inhibitors a predisposing factor? Clin Endocrinol (Oxf) 2020; 92(3):258–265.
35. Abdel-Rahman O, Fouad M. Risk of thyroid dysfunction in patients with solid tumors treated with VEGF receptor tyrosine kinase inhibitors: a critical literature review and meta-analysis. Expert Rev Anticancer Ther 2014; 14(9):1063–1073.
36. Kurimoto C, Inaba H, Ariyasu H, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 2020; 111(5):1468–1477.
37. Barroso-Sousa R, Ott PA, Hodi FS, Kaiser UB, Tolaney SM, Min L. Endocrine dysfunction induced by immune checkpoint inhibitors: practical recommendations for diagnosis and clinical management. Cancer 2018; 124(6):1111–1121.
38. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet Med 2019; 36(9): 1075–1081.
39. Gaudy C, Clévy C, Monestier S, et al. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care 2015; 38(11):e182–e183.
40. Ishikawa K, Shono-Saito T, Yamate T, et al. A case of fulminant type 1 diabetes mellitus, with a precipitous decrease in pancreatic volume, induced by nivolumab for malignant melanoma: analysis of HLA and CTLA-4 polymorphisms. Eur J Dermatol 2017; 27(2): 184–185.
41. de Filette JMK, Pen JJ, Decoster L, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 2019; 181(3):363–374.
42. Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab 2018; 103(9):3144–3154.
43. Ferrari SM, Fallahi P, Elia G, et al. Autoimmune endocrine dysfunctions associated with cancer immunotherapies. Int J Mol Sci 2019; 20(10):2560.
44. Grouthier V, Lebrun-Vignes B, Moey M, et al. Immune checkpoint inhibitor-associated primary adrenal insufficiency: WHO VigiBase report analysis. Oncologist 2020; 25(8):696–701.
45. Harsch IA. Hypothesis: does adrenalitis caused by immune checkpoint-inhibitors put melanoma patients at an elevated risk for recurrence? J Immunother Cancer 2019; 7(1):166.
46. Piranavan P, Li Y, Brown E, Kemp EH, Trivedi N. Immune checkpoint inhibitor-induced hypoparathyroidism associated with calcium-sensing receptor-activating autoantibodies. J Clin Endocrinol Metab 2019; 104(2):550–556.
47. Falcao CK, Cabral MCS, Mota JM, et al. Acquired lipodystrophy associated with nivolumab in a patient with advanced renal cell carcinoma. J Clin Endocrinol Metab 2019; 104(8):3245-3248.
48. Gunjur A, Klein O, Kee D, Cebon J. Anti-programmed cell death protein 1 (anti-PD1) immunotherapy induced autoimmune polyendocrine syndrome type II (APS-2): a case report and review of the literature. J Immunother Cancer 2019; 7(1):241.
49. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update [published correction appears in J Clin Oncol 2022; 40(3):315]. J Clin Oncol 2021; 39(36):4073–4126.
50. Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, version 1.2022: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022; 20(4):387–405.
51. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021; 9(6):e002435.
52. Yuen KCJ, Samson SL, Bancos I, et al. American Association of Clinical Endocrinology disease state clinical review: evaluation and management of immune checkpoint inhibitor-mediated endocrinopathies: a practical case-based clinical approach. Endocr Pract 2022; 28(7):719–731.
53. Min L, Hodi FS, Giobbie-Hurder A, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumabrelated hypophysitis: a retrospective cohort study. Clin Cancer Res 2015; 21(4):749–755.
54. Albarel F, Gaudy C, Castinetti F, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol 2015; 172(2):195–204.
55. Foster CC, Couey MA, Kochanny SE, et al. Immune-related adverse events are associated with improved response, progression-free survival, and overall survival for patients with head and neck cancer receiving immune checkpoint inhibitors. Cancer 2021; 127(24): 4565–4573.
56. Martini DJ, Goyal S, Liu Y, et al. Immune-related adverse events as clinical biomarkers in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Oncologist 2021; 26(10):e1742–e1750.
Advertisement
Pheochromocytoma case underscores the value in considering atypical presentations
Advocacy group underscores need for multidisciplinary expertise
A reconcilable divorce
A review of the latest evidence about purported side effects
High-volume surgery center can make a difference
Advancements in equipment and technology drive the use of HCL therapy for pregnant women with T1D
Patients spent less time in the hospital and no tumors were missed
A new study shows that an AI-enabled bundled system of sensors and coaching reduced A1C with fewer medications