Insights indicate that treatment may be beneficial beyond MSI-H tumors
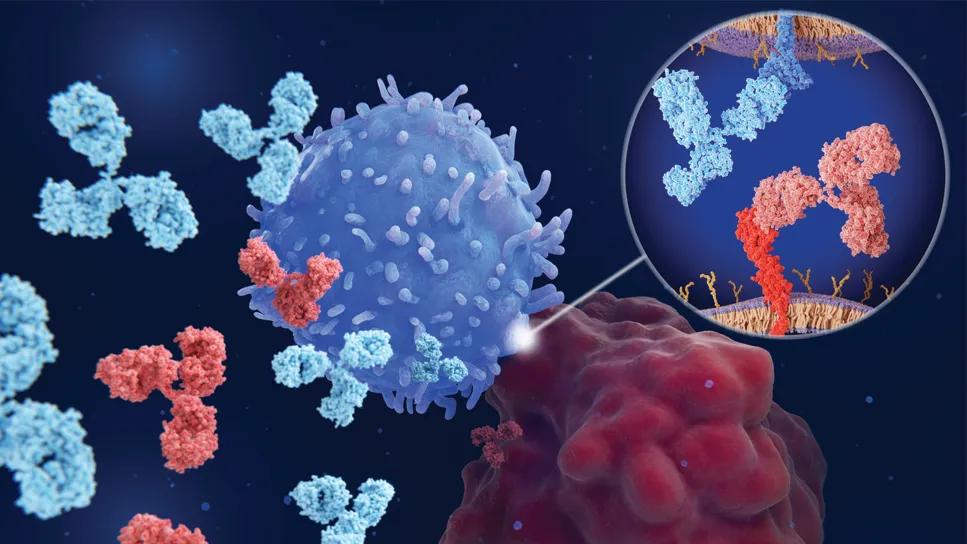
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6af837e9-04ae-4842-9137-c8230c5a942c/new-colorectal-cancer-immunotherapy-1408900936)
Immune checkpoint inhibitor illustration
Pictured: Immune checkpoint inhibitors at work. An immune cell (darkest blue) uses its PD-1 immune checkpoint protein (medium blue, inset) to attempt to recognize and bind to the PD-L1 immune checkpoint protein (medium red, inset) of a cancer cell (darkest red). The interaction is blocked by immune checkpoint inhibitors (lighter blue and lighter red, inset).
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Below is an abbreviated version of an article that is being reprinted from Lerner Research Institute (LRI).
Immune checkpoint inhibitors greatly improved survival rates for patients living with metastatic MSI-H colorectal cancer, according to analysis of 19,000 patients. A team of researchers from the lab of Stephanie Schmit, PhD, MPH, at LRI’s Genomic Medicine Institute reported the findings In JAMA Network Open.
Notably, the researchers also found certain conditions that may improve the therapy’s effectiveness against MSS tumors under certain conditions.
In 2017, the FDA approved six immune checkpoint inhibitors to be used for treatment of colorectal cancer patients with microsatellite instable (MSI-H) tumors. The therapies had great success as a treatment for metastatic colorectal cancer in clinical trials. Building on this work, the study team used an international database from healthcare technology company Flatiron Health to learn how a large group of patients responded to the treatment in typical, routine clinical practice.
Dr. Matejcic, who works in Dr. Schmit’s lab, teamed up with co-first author Shahla Bari, MD, an oncologist who formerly worked at Moffitt, to analyze electronic health records of almost 19,000 individuals who received treatment for their colorectal cancer between 2013 and 2019. He analyzed the records to identify factors that correlated with a specific treatment outcome for patients treated with or without the immune checkpoint inhibitors.
The team’s results supported the earlier clinical trial findings, showing that immune checkpoint inhibitors greatly improved survival rates for patients living with MSI-H metastatic colorectal cancer.
Advertisement
Interestingly, they also identified different factors that could influence how well the treatments worked in patients with microsatellite stable (MSS) tumors. MSS tumors are generally not very responsive to the immune checkpoint inhibitor therapy so the FDA currently approves the treatment for MSI-H tumors.
“While most of the individuals with MSS tumors we observed responded poorly, there actually were some MSS tumors with durable responses,” Dr. Schmit says. “Factors like enzyme levels, microbiome activity, additional medications and more each played a small role in determining a MSS tumor’s response to immune checkpoint therapy, though we can’t make any specific recommendations until we do more research.”
Many physicians prescribe chemotherapy or immunotherapy based on their patients’ tumor status. Because individuals with MSS tumors did not respond as well to the therapy in clinical trials, physicians are less likely to prescribe immune checkpoint inhibitors to these patients. The Schmit Lab’s additional findings indicate there are more options.
“Our study may provide new guidelines/changes in guidelines for MSS tumors that have generally been unresponsive to immune checkpoint inhibitors in clinical trials, although our findings need to be replicated in larger studies,” Dr. Matejcic says. “We hope that our findings will contribute to improving the survival outcome of MSS colorectal cancer patients who are currently unresponsive to immunotherapy.”
Read the full-length version of this article.
Advertisement
Advertisement
Key points highlight the critical role of surveillance, as well as opportunities for further advancement in genetic counseling
Simple score uses clinical factors to identify patients who might benefit from earlier screening
Radiation to the pelvis from cancer treatment made the traditional treatment path unavailable
Surgery, chemotherapy improve outcomes among EOCRC patients
ctDNA should be incorporated into care to help stratify risk pre-operatively and for post-operative surveillance
Unique molecular features found in young-onset disease
Greater awareness among young patients is needed
Although the number of surgeon-scientists in colorectal surgery is small, a Cleveland Clinic colorectal surgeon-scientist shares his thoughts on what surgeon-scientists bring to the table