Cardiac manifestations of this autoimmune disorder can be hidden but deadly
By Preethi Mani, MD; Danny Gonzalez, MD; Soumya Chatterjee, MD, MS; and Michael D. Faulx, MD
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
This article is reprinted from the Cleveland Clinic Journal of Medicine (2019;86[10]:685-695).
Autoimmune rheumatic diseases increase the risk of cardiovascular disease. In rheumatoid arthritis and systemic lupus erythematosus, the risk is driven primarily by the inflammatory milieu, leading to accelerated coronary and cerebrovascular atherosclerosis independent of traditional atherosclerotic risk factors.1-3 The extent of cardiovascular involvement in other rheumatologic diseases has been less well characterized but is an area of growing interest.
In this review, we focus on the cardiovascular complications of systemic sclerosis and review recommendations for monitoring these patients in clinical practice.
Systemic sclerosis is an autoimmune rheumatic disease characterized by excessive extracellular matrix deposition leading to diffuse fibrosis, endothelial dysfunction and microvascular injury. It is most common in North America, Southern Europe and Australia,4,5 and it affects women more than men in ratios ranging from 3:1 to 14:1.6 The mean age at diagnosis is around 50.
The disease can affect the lungs (interstitial lung disease and pulmonary hypertension), the heart, the kidneys and the gastrointestinal tract.
Systemic sclerosis has two main subtypes: limited cutaneous systemic sclerosis (formerly called CREST syndrome) and diffuse cutaneous systemic sclerosis. The limited cutaneous subtype is characterized by tightening of the skin of the distal extremities (below the elbows and knees) and face, while diffuse cutaneous systemic sclerosis can manifest as more extensive skin tightening also involving proximal extremities and the trunk. Both subtypes can have an effect on the cardiovascular system.
Advertisement
Some cardiovascular risk factors such as dyslipidemia, diabetes mellitus and high body mass index are less common in patients with systemic sclerosis than in patients with rheumatoid arthritis, while rates of arterial hypertension, smoking, chronic obstructive pulmonary disease, osteoporosis and neoplasms are similar between the two groups.7
Overt cardiac involvement in systemic sclerosis is associated with a mortality rate of up to 70% over five years,8,9 and about one-fourth of deaths in patients with systemic sclerosis are from cardiac causes.10,11 Studies in Europe10,12 showed that many patients with systemic sclerosis have cardiac involvement detectable by MRI even if they do not have clinical disease. Pulmonary arterial hypertension is a complication of both subtypes of systemic sclerosis and portends a higher risk of death.8
Thus, it is critical for clinicians to understand the potential comorbid conditions associated with systemic sclerosis, particularly the cardiovascular ones, and to work closely with cardiologists to help optimize the evaluation and management.
Microvascular disease in systemic sclerosis is primarily driven by endothelial cell activation and injury, leading to overexpression of adhesion molecules, recruitment of immune cells, intimal fibrosis and fibroblast proliferation (Figure 1).13
Abnormal vasoreactivity, a consequence of an imbalance between endothelium-derived vasoconstrictors and vasodilators, defective angiogenesis and endothelial injury, leads to tissue ischemia and vascular endothelial growth factor expression, which initiates injury and fibrosis in the myocardium and other organs.14-17 Fibrosis involves the myocardium, pericardium and conduction system.13,18
Advertisement
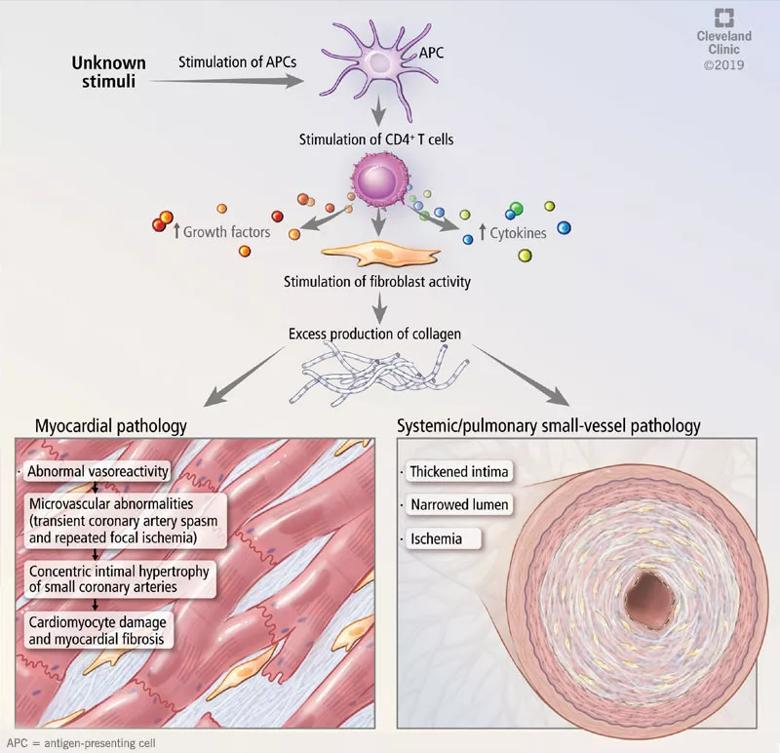
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/dfc5519f-1471-41c4-97f0-67f795fa03b9/19-HRT-6557-cardiac-involvement-systemic-sclerosis-800x772_jpg)
Figure 1. Mechanisms of cardiac and vascular involvement in systemic sclerosis.
Myocardial involvement in systemic sclerosis is thought to be due mainly to abnormal vasoreactivity and microvascular abnormalities such as transient coronary artery spasm leading to repeated focal ischemia.19,20 Abnormal vasoreactivity has been demonstrated during cardiac catheterization21: while mean coronary sinus blood flow in systemic sclerosis patients was normal at rest, vasodilator reserve was significantly reduced in patients with diffuse cutaneous systemic sclerosis after maximal vasodilation with dipyridamole. Additionally, endomyocardial biopsy showed fibrosis and concentric intimal hypertrophy with normal epicardial coronary arteries.21
More research into other mechanisms of cardiovascular disease in systemic sclerosis is needed to allow for better preventive care for these patients.
Systemic sclerosis can be associated with World Health Organization (WHO) groups 1, 2, 3 and 4 pulmonary hypertension. WHO group 1, called pulmonary arterial hypertension (PAH), is one of the most common cardiac complications of systemic sclerosis, with a reported prevalence as high as 12%.22 Systemic sclerosis-associated PAH carries a high mortality rate, with a mean survival of only three years.23
With advances in treatments for other complications of systemic sclerosis, the percentage of systemic sclerosis patients who die of PAH has increased from 6% to 33%.24
Compared with patients with idiopathic PAH, those with systemic sclerosis get less of a response from therapy and have poorer outcomes despite lower mean pulmonary artery pressures and similar reductions in cardiac index. However, recent studies have suggested that with aggressive treatment, patients with systemic sclerosis-related PAH can achieve outcomes similar to those with idiopathic PAH.25 Thus, recognizing this condition early is imperative.
Advertisement
PAH is defined as the combination of all of the following26:
Other causes of pulmonary hypertension — such as interstitial lung disease, chronic pulmonary thromboembolic disease and left heart disease — must be excluded.24,27
Remodeling in the pulmonary arteries. The events that lead to PAH in systemic sclerosis remain unclear but are believed to involve initial inflammation or endothelial injury that leads to a disequilibrium between proliferative mediators and antiproliferative vasodilators. This disequilibrium, along with endothelial dysfunction, causes an obliterative vasculopathy in the pulmonary artery branches and arterioles. Sympathetic overactivity, hypoxemia and ischemia-reperfusion injury additionally promote vascular proliferation, fibrosis and remodeling, leading to increased pulmonary vascular resistance, PAH and increased right ventricular pressures.23,27
The subtype of systemic sclerosis is an important factor in the development and progression of PAH. PAH appears to be the major cause of death in limited cutaneous systemic sclerosis, while interstitial lung disease is the major cause of death in diffuse cutaneous systemic sclerosis.28
A late complication of systemic sclerosis. Data from the South Australian Scleroderma Registry29 revealed that PAH tends to be a late complication of systemic sclerosis, occurring around 20 years after disease onset. In this study of 608 patients, no patient with diffuse cutaneous systemic sclerosis developed PAH.
Advertisement
Systemic sclerosis-related PAH initially follows an indolent course with few symptoms until right ventricular function deteriorates. Early in the disease, patients may experience nonspecific symptoms of fatigue, lightheadedness and dyspnea on exertion.23 As it progresses, they tend to have worsening dyspnea and may experience exertional syncope, palpitations and chest pain.
Physical findings may suggest elevated right ventricular pressure and right ventricular failure; these include a loud P2, a prominent jugular a wave, a tricuspid regurgitant murmur, jugular venous distention and lower-extremity edema.27
Screening for PAH in systemic sclerosis.Significant signs and symptoms usually occur late in the disease, so it’s important to appropriately screen patients who are at risk to allow for aggressive treatment.
Doppler echocardiography is recommended by European and American guidelines to screen for PAH in patients who have systemic sclerosis, and most agree that screening is appropriate even if the patient lacks symptoms.30 European consensus documents recommend that transthoracic echocardiography be done annually for the first five years of disease and be continued every year in patients at high risk (i.e., those with anticentromere antibodies, anti-Th/To antibodies or interstitial lung disease). Patients not at high risk of developing pulmonary hypertension should also have regular transthoracic echocardiography, though the exact timing is not defined.31
Worrisome features on echocardiography in asymptomatic patients should be followed up with right heart catheterization to assess mean right ventricular pressure. These include32:
Although echocardiography is the most common form of screening, it gives only an estimate of right ventricular systolic pressure, which is imprecise. Other noninvasive markers are helpful and necessary to appropriately screen this population, including:
An algorithm for PAH screening. The DETECT study36 enrolled patients at increased risk who had had systemic sclerosis longer than three years and a DLCO less than 60%. The investigators developed a two-step algorithm to determine which patients should be referred for right heart catheterization to try to detect PAH earlier while minimizing missed diagnoses and optimizing the use of invasive diagnostic right heart catheterization.
The first step was to assess serum values of anticentromere antibodies, NT-proBNP and urate, and clinical features (telangiectasias), forced vital capacity, and electrocardiographic changes of right axis deviation to derive a prediction score. The second step was to assess surface echocardiographic features of the right atrial area and tricuspid regurgitation velocity.
This approach led to right heart catheterization in 62% of patients and was associated with a false-negative rate of 4%. Importantly, of the patients with PAH, 1 in 5 had no symptoms, and 33% had tricuspid regurgitation velocity less than 2.8 m/s. No single measurement performed well in isolation in this study.37
Thus, we recommend that, in addition to routine surface echocardiography, a multimodal approach be used that includes laboratory testing, clinical features and electrocardiographic findings when screening this high-risk patient population.
Although macrovascular disease has not typically been regarded as a significant systemic feature in systemic sclerosis, myocardial infarction and stroke are more common in patients with systemic sclerosis than in controls.38,39
Coronary artery disease in systemic sclerosis.Man et al38 reported that the incidence of myocardial infarction in patients with systemic sclerosis was 4.4 per 1,000 persons per year, and the incidence of stroke was 4.8 per 1,000 persons per year, compared with 2.5 per 1,000 persons per year for both events in healthy controls matched for age, sex and time of entry.
The Australian Scleroderma Cohort Study39 found a threefold higher prevalence of coronary artery disease in systemic sclerosis patients than in controls after factoring in traditional risk factors.
Aviña-Zubieta et al,40 in a cohort of 1,239 systemic sclerosis patients, estimated a hazard ratio of 3.49 for myocardial infarction and 2.35 for stroke compared with age- and sex-matched controls. Not all events were related to macrovascular atherosclerosis — vasospasm and microvascular ischemia may have played significant roles in the etiology of clinical manifestations.
Studies of coronary atherosclerosis in systemic sclerosis are limited. An autopsy study41 of 58 patients with systemic sclerosis and 58 controls matched for age, sex and ethnicity found that the prevalence of atherosclerosis of small coronary arteries and arterioles was significantly higher in systemic sclerosis patients than in controls (17% vs. 2%, P < 0.01). However, the prevalence of medium-vessel coronary atherosclerosis was similar (48% vs. 43%).
Why patients with systemic sclerosis develop atherosclerosis has not been determined. Traditional risk factors such as hypertension, dyslipidemia, diabetes mellitus and obesity are typically no more prevalent in systemic sclerosis patients than in controls38,42 and thus do not explain the increased risk of atherosclerotic cardiovascular disease. There is some evidence that novel markers of atherosclerotic risk, such as homocysteine,43 lipoprotein[a]44 and oxidized low-density lipoprotein,45 are more prevalent in systemic sclerosis, but these results have not been substantiated in more extensive studies.
Peripheral artery disease. It remains unclear whether peripheral artery disease is more prevalent in systemic sclerosis patients than in controls.
Individual studies have shown mixed results in comparing carotid artery stenosis between systemic sclerosis patients and controls using carotid duplex ultrasonography,46 the ankle-brachial index,46-48 carotid intima-media thickness49-54 and brachial flow-mediated dilation.51,53,55-58 A meta-analysis found that the carotid intima and media are significantly thicker in systemic sclerosis patients than in controls,59 and the magnitude of difference is similar to that in other groups at increased cardiovascular risk, such as those with rheumatoid arthritis, diabetes and familial hypercholesterolemia.60-63
A meta-analysis of brachial artery findings showed significantly lower flow-mediated dilation in systemic sclerosis patients than in controls.64
Overall, given the inconsistency of study results, systemic sclerosis patients should be screened and managed like other patients with peripheral artery disease, but clinicians should be aware that there may be a higher risk of peripheral artery disease in these patients.
Many patients with systemic sclerosis have right ventricular dysfunction as a consequence of PAH.65 It is important to detect diastolic dysfunction in this population, as it may be an even stronger predictor of death than pulmonary hypertension on right heart catheterization (hazard ratio of 3.7 vs. 2.0).66
Fewer patients have left ventricular dysfunction. In a multicenter study of 570 systemic sclerosis patients, only 1.4% had left ventricular systolic dysfunction on echocardiography, though 22.6% had left ventricular hypertrophy and 17.7% had left ventricular diastolic dysfunction.67 In the European League Against Rheumatism (EULAR) database, the prevalence of reduced left ventricular ejection fraction was 5.4%.68
Though traditional echocardiographic screening suggests the prevalence of left ventricular dysfunction in systemic sclerosis patients is low, cardiac MRI may be more sensitive than echocardiography for detecting subclinical myocardial involvement. Cardiac MRI has been shown to detect evidence of myocardial pathology (increased T2 signal, left ventricular thinning, pericardial effusion, reduced left and right ventricular ejection fraction, left ventricular diastolic dysfunction and delayed myocardial contrast enhancement) in up to 75% of systemic sclerosis cases studied.69
Patients with systemic sclerosis should already be undergoing echocardiography every year to screen for PAH, and screening should also include tissue Doppler imaging to detect forms of left and right ventricular systolic and diastolic dysfunction that may not be clinically apparent.
Though cardiac MRI can provide useful additional information, it is not currently recommended for routine screening in patients with systemic sclerosis.
Patients with systemic sclerosis are prone to arrhythmias due to both conduction system fibrosis and myocardial damage.
Arrhythmias accounted for 6% of the deaths in the EULAR Scleroderma Trials and Research (EUSTAR) database.11
In the Genetics Versus Environment in Scleroderma Outcome Study (GENISOS),70 250 patients who had had systemic sclerosis for at least three years were studied for approximately six years, during which there were 52 deaths, 29 of which were directly attributable to systemic sclerosis. Multivariable Cox modeling showed that seven variables predicted mortality:
The hazard ratio for death in patients with arrhythmias in this model was 2.18 (95% CI, 1.05-4.50, P = 0.035). Thus, finding arrhythmias in systemic sclerosis patients can provide important prognostic information.
While resting electrocardiography in patients with systemic sclerosis most commonly shows sinus rhythm, 24-hour electrocardiographic monitoring has revealed nonsustained supraventricular and ventricular arrhythmias in a significant percentage.71,72 Although difficult to quantify in routine practice, parameters controlled by the autonomic nervous system including heart rate variability and heart rate turbulence have been shown to be impaired in systemic sclerosis, and these measures are associated with an increased risk of malignant arrhythmias and sudden cardiac death.73,74
Conduction abnormalities. Conduction abnormalities occur in one-fifth to one-third of patients with systemic sclerosis.75,76 The most common finding is left bundle branch block, followed by first-degree atrioventricular block. High-degree atrioventricular block is uncommon,76 though a few case reports of complete heart block thought to be related to systemic sclerosis have been published.77-79 An autopsy study showed that the conduction system is relatively spared from myocardial changes seen in systemic sclerosis patients, so it is speculated that the conduction disturbances are a consequence of damaged myocardium rather than damage to conduction tissue.80
Given the array of electrophysiologic abnormalities that systemic sclerosis patients can have, it is critical to monitor all patients with routine (annual or biannual) electrocardiography; to take possible arrhythmia-related symptoms seriously; and to evaluate them with further workup such as Holter monitoring for 24 hours or longer, event monitoring, exercise testing or tilt-table testing.
Pericardial disease is clinically apparent in 5% to 16% of patients with systemic sclerosis81; patients with limited cutaneous systemic sclerosis have more pericardial disease than those with diffuse cutaneous systemic sclerosis (30% vs. 16%).82 Forty-one percent of systemic sclerosis patients have been shown to have pericardial effusion by echocardiography,81 but the effusions are typically small and rarely cause tamponade, though tamponade is associated with a poor prognosis.
Large pericardial effusions can develop before skin thickening and diagnosis of systemic sclerosis.81,83,84 Thus, systemic sclerosis should be considered in patients with pericardial effusions of unknown etiology.
In a small study,85 the pericardial fluid in systemic sclerosis was typically exudative, with lactate dehydrogenase greater than 200 U/L, a fluid-serum lactate dehydrogenase ratio greater than 0.6, and a fluid-serum total protein ratio greater than 0.5.
Pericardial effusion can be a sign of impending scleroderma renal crisis,86 so renal function should be carefully monitored in systemic sclerosis patients with pericardial effusion. Constrictive pericarditis and restrictive cardiomyopathy can rarely occur in systemic sclerosis and may more commonly present with symptoms.
Pericardial disease in systemic sclerosis should be treated in a standard fashion with nonsteroidal anti-inflammatory drugs. Corticosteroids are generally of limited benefit and should be avoided, especially in the setting of scleroderma renal crisis.81
Based on limited studies, the prevalence of significant valvular heart disease does not seem to be higher in systemic sclerosis patients than in the general population. While patients with systemic sclerosis and CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly and telangiectasia) have been found to have a higher frequency of mitral valve prolapse and mild mitral regurgitation,87,88 these abnormalities do not often progress in severity and thus have limited clinical significance.
It is important for physicians caring for patients with systemic sclerosis to be aware of its most common cardiac manifestations, including left and right ventricular systolic and diastolic dysfunction, pulmonary hypertension, conduction abnormalities, arrhythmias and cardiomyopathy.
Look for volume overload. On clinical examination, assess for clinical markers of volume overload such as distended neck veins, peripheral edema or an abnormal blood pressure response to the Valsalva maneuver. These findings should prompt measurement of NT-proBNP89 and may warrant prescription of a diuretic.
Electrocardiography to investigate arrhythmias. Electrocardiography should be done if patients describe symptoms of palpitations, and should also include continuous rhythm monitoring with Holter or event monitoring, depending on the frequency of symptoms. Otherwise, patients should routinely undergo electrocardiography once or twice a year.
Q waves are common in patients with systemic sclerosis (especially the diffuse cutaneous form), notably in the precordial leads, and can occur without coronary artery disease.90 Symptoms such as presyncope should be further investigated with Holter monitoring and tilt-table testing.
Assess, modify traditional risk factors. Subclinical atherosclerosis as detected by carotid intima-media thickness is as common in systemic sclerosis as in rheumatoid arthritis.61 However, traditional risk indices such as SCORE (Systematic Coronary Risk Evaluation), QRISK2, and the American College of Cardiology/American Heart Association indices may underestimate risk in patients with systemic sclerosis.
Echocardiography to detect PAH. At this time, guidelines for monitoring for cardiovascular manifestations in systemic sclerosis patients are limited. The only well-defined ones are European consensus guidelines, which suggest annual transthoracic echocardiography for the first five years after systemic sclerosis is diagnosed and continued annual screening in patients at risk of developing PAH.31
We support this strategy, with annual screening for the first five years followed by surveillance echocardiography every two to three years unless there is a high risk of PAH. Specific attention should be paid to right ventricular diastolic function, right atrial volume and right ventricular myocardial performance index.
Emerging data suggest that the addition of global longitudinal strain of ventricles to routine echocardiography can help detect subclinical cardiac risk.94 Although further study is needed into the predictive value of global longitudinal strain, it is a low-cost and noninvasive addition to standard echocardiography that can help guide risk stratification, and thus we recommend that it be part of the echocardiographic examination for all systemic sclerosis patients.
In addition to screening for PAH with echocardiography, we recommend obtaining baseline pulmonary function tests, including DLCO, when systemic sclerosis is diagnosed, with repeat testing annually.
MRI. While echocardiography is the gold standard for monitoring systemic sclerosis patients, cardiovascular MRI may have a role in identifying those at higher risk of dangerous arrhythmias such as ventricular tachycardia and ventricular fibrillation. In addition to assessing ventricular function, MRI can detect myocardial inflammation, ischemia and fibrosis that may predispose to development of ventricular tachycardia or fibrillation.95 Variables such as T1/T2 mapping, extracellular volume fraction, T2 signal rati, and early versus late gadolinium enhancement can help identify patients who had past ventricular tachycardia or fibrillation.96
Finding an increased risk of arrhythmias may prompt a conversation between the patient and the physician about the need for an implantable cardiac defibrillator.
If cardiac MRI is available and is reimbursed by the patient’s insurance carrier, physicians should strongly consider obtaining at least one baseline scan in systemic sclerosis patients to identify those at risk of highly fatal arrhythmias.
Teamwork is needed. Systemic sclerosis has not traditionally been associated with cardiovascular disease to the extent of other rheumatic conditions, but the cardiovascular system can be affected in various ways that can ultimately lead to an early death. These manifestations may be hidden for long periods, and overt clinical disease portends a poorer prognosis.
It is essential for general and subspecialty cardiologists to understand the broad spectrum of organ system involvement that can affect systemic sclerosis patients and to tailor their investigation and management recommendations accordingly. By designing a multidisciplinary approach to the treatment of systemic sclerosis patients, physicians can help to optimize cardiovascular risk modification in this vulnerable population.
This article is reprinted from the October 2019 issue of Cleveland Clinic Journal of Medicine (2019;86[10]:685-695).
All authors are with Cleveland Clinic. Drs. Mani and Gonzalez are fellows in the Department of Cardiovascular Medicine. Dr. Chatterjee is a staff physician in the Department of Rheumatic and Immunologic Diseases. Dr. Faulx is a staff physician in the Section of Clinical Cardiology, Department of Cardiovascular Medicine.
Advertisement

How Cleveland Clinic is using and testing TMVR systems and approaches

NIH-funded comparative trial will complete enrollment soon

How Cleveland Clinic is helping shape the evolution of M-TEER for secondary and primary MR

Optimal management requires an experienced center

Safety and efficacy are comparable to open repair across 2,600+ cases at Cleveland Clinic

Why and how Cleveland Clinic achieves repair in 99% of patients

Multimodal evaluations reveal more anatomic details to inform treatment

Insights on ex vivo lung perfusion, dual-organ transplant, cardiac comorbidities and more