Nivolumab, CV301 and systemic chemotherapy trial
A 62-year-old woman presented to Cleveland Clinic Cancer Center with bloody diarrhea associated with left lower abdominal cramps and pain. Oncologist Suneel Kamath, MD, ordered a CT scan that showed masses in the liver and in the colon. The diagnosis: stage 4 colorectal cancer metastasized to the liver. Instead of the standard treatment, Dr. Kamath opted for a leading-edge clinical trial.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
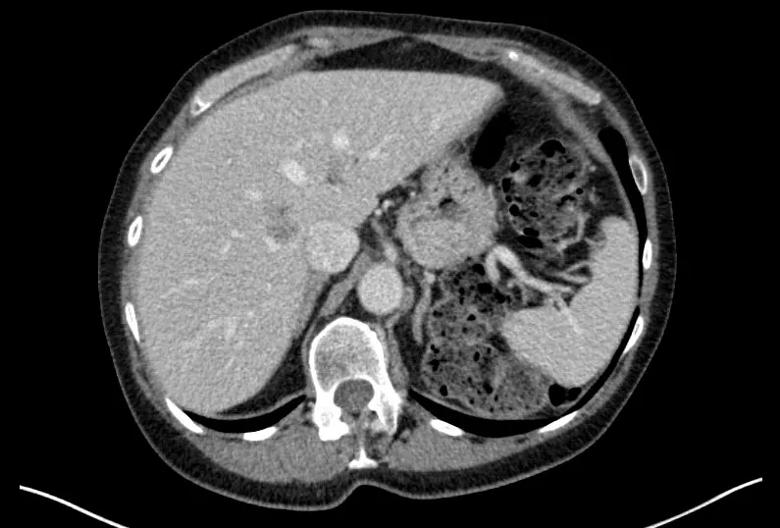
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a3319d77-140d-491b-a34c-ab9845e15542/Before1-1_png)
“We decided to pursue a novel clinical trial that combined immunotherapy, an anti-tumor vaccine and chemotherapy to shrink her colon tumor and the liver tumors,” Dr. Kamath says. “Surgery to remove all sites of the cancer in the liver and colon would come later.”
Fortunately, Cleveland Clinic was taking part in HCRN 1218: A Phase II Trial of Perioperative CV301 Vaccination in Combination with Nivolumab and Systemic Chemotherapy for Resectable Hepatic-Limited Metastatic Colorectal Cancer. The double-arm, randomized phase 2 clinical trial pairs nivolumab, an anti-PD-1 monoclonal antibody, with an investigational vaccine, CV301. Together, this treatment stimulates an immune response to two protein markers of colon cancer, CEA and MUC1. The immune response generated by nivolumab, which can sometimes not be as specific to a particular tumor, gets more targeted towards these two markers of the colon cancer with the addition of CV301.
Half of patients receive chemotherapy and immunotherapy, the other half receive chemotherapy, immunotherapy and the vaccine. The 62-year-old patient received all three.
“It’s truly an amazing study. The fact that Cleveland Clinic is one of the trial sites shows that innovative treatments are valued here,” says Dr. Kamath. He also notes that patients should be prepared to hear about the latest investigational treatment options when they come to a place like Cleveland Clinic. “This patient was more than willing to try a cutting-edge treatment.”
The patient started the pioneering treatment plan in September 2019. Four months later — January 2020 — she had surgery to remove all sites of the cancer in the liver and the colon.
Advertisement
“Amazingly, when the colorectal and liver surgeons removed the spots where the colon tumor and liver metastases had been, there was no tumor left,” Dr. Kamath says. “The immunotherapy, chemotherapy and vaccine had killed everything. We struggled to even find the tumor.”
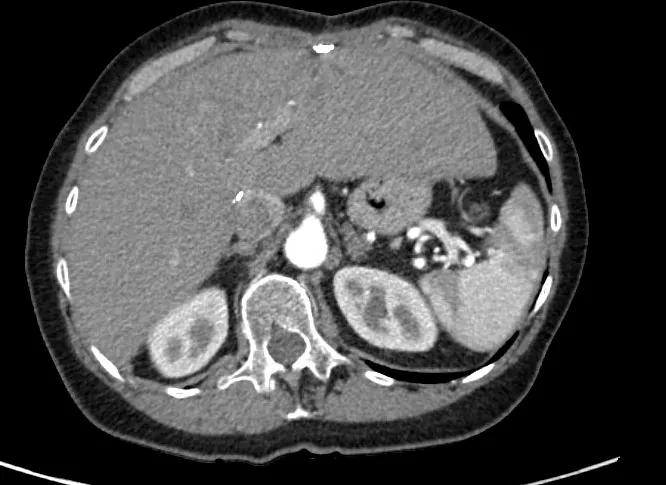
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2a05befe-8160-4724-8f42-f34cccd58702/After1-1_png)
CT liver after treatment.
Because the treatment for this complex case involved a clinical trial, immunotherapy, chemotherapy, and surgery, Dr. Kamath credits Cleveland Clinic surgeons Federico Aucejo, MD, and Michael Valente, MD, for their multidisciplinary teamwork.
“Without Cleveland Clinic’s emphasis on multidisciplinary care, the patient would have had to undergo two different surgeries,” Dr. Kamath notes. “Having world-class expertise from two surgeons to remove tumors that were previously unresectable is invaluable.”
Dr. Aucejo is no stranger to innovative treatment. He’s been involved in a cutting-edge surgical treatment option that leverages the liver’s regenerative capacity, which has shown promise for patients with previously unresectable liver metastases from colorectal cancer.
After surgery, Dr. Kamath’s patient continued chemotherapy and immunotherapy. Now, more than one year later, she’s still cancer free. The clinical trial will track overall survival at three years as a primary outcome.
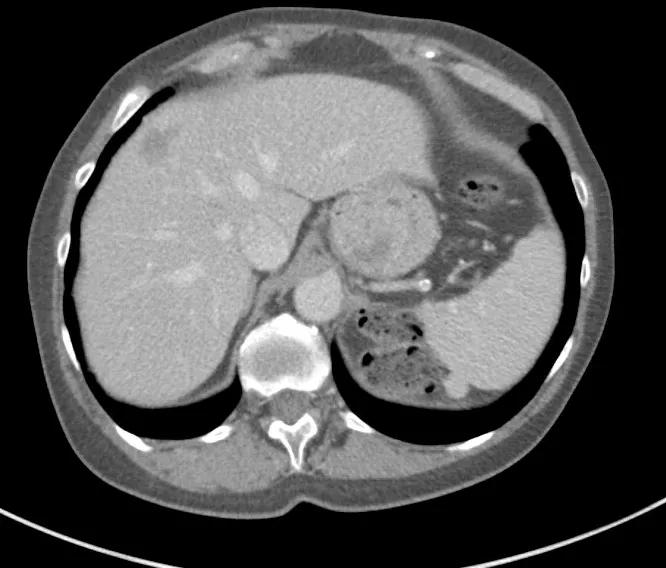
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/98e40ebc-ec78-47e1-947c-a3dff3d3a629/Before2-1_png)
CT liver before treatment.
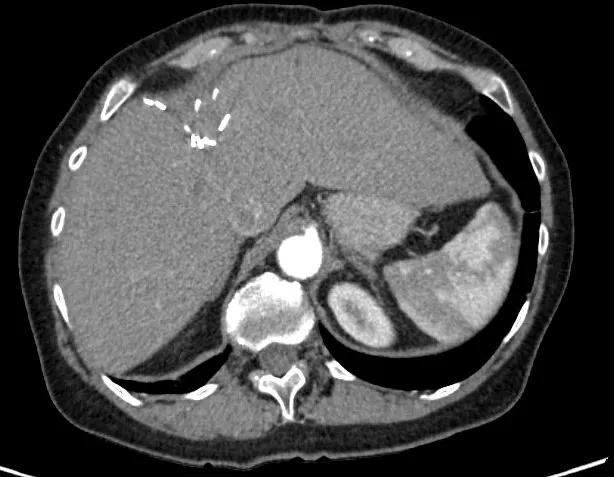
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/3d64f9cf-6e57-40ee-9eca-119c54f0751e/After2-1_png)
CT liver after treatment.
“This healthy, fit woman has continued to live her life to the fullest, even during treatment,” says Dr. Kamath, noting that the side effects were minor and well-tolerated. “And that’s what our work is all about. It’s more than just eradicating the cancer, but ensuring our patients are able to continue doing the things they love.”
“This case has given us all lots of promise and optimism,” Dr. Kamath says. “By pursuing novel therapies combined with multidisciplinary care, we can continue instilling hope in the lives of our patients and their families.”
Advertisement
Advertisement

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists