Immune checkpoint inhibitor and oncolytic viral immunotherapy produce synergistic effect
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/b8cf08da-3ef3-468a-9fbe-c93512aaedd3/19-CNR-4146-MMC-650x450-1_jpg)
19-CNR-4146 MMC 650×450
Cleveland Clinic Cancer Center physicians achieved remission in two patients with refractory advanced Merkel cell carcinoma (MCC) by using a novel treatment that combines two forms of immunotherapy.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Their report, published recently in Annals of Oncology, is the first account of successful treatment of the disease with talimogene laherparepvec (TVEC) and pembrolizumab in multiple individuals.
MCC is a rare and aggressive form of skin cancer typically seen in patients older than age 50 who are immunocompromised or immunosuppressed. Few treatments exist for MCC patients whose disease becomes refractory to standard therapies. Inhibitors of the programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) axis such as pembrolizumab have been studied as treatment for advanced MCC, but most responses are not durable.
TVEC is a first-in-class oncolytic viral immunotherapy that is U.S. Food and Drug Administration (FDA)-approved to treat unresectable melanoma, although it is not currently approved for MCC. It is a genetically modified herpes simplex 1 virus that encodes human granulocyte-macrophage colony-stimulating factor to enhance dendritic cell antigen presentation. The immune checkpoint inhibitor pembrolizumab earned FDA approval for MCC use in December 2018. Both TVEC and pembrolizumab are administered intratumorally by injection.
“MCC is similar to melanoma, and therapy with TVEC has been studied in that disease,” says plastic surgeon and researcher Brian Gastman, MD, a co-author of the case report and the Medical and Surgical Director of Cleveland Clinic’s Melanoma and High-risk Skin Cancer Program. “With TVEC, a genetically manipulated virus is injected into cancer cells and it replicates and explodes. The combination of TVEC and pembrolizumab is synergistic.”
Advertisement
Previous research has shown that cancer patients who are resistant to PD-1/PD-L1 inhibition therapy often lack CD8+ T cells in the tumor microenvironment, likely negating the PD-1 blockade’s effectiveness. TVEC’s optimization to attract immune cells may alter the tumor microenvironment and increase CD8+ T cell infiltration, inducing an enhanced systemic response.
The case report by Dr. Gastman and his colleagues documents the courses of two patients: a 76-year-old white male with a T2N1a MCC on his right leg (Patient 1) and a 69-year-old white male with an MCC of the right neck (Patient 2).
Patient 1 underwent resection but not radiation therapy due to delayed wound healing. After regional and systemic recurrence, his subsequent treatment included pembrolizumab, etoposide/carboplatin, TVEC alone, ipilimumab alone, and avelumab in combination with TVEC. After treatment with TVEC plus pembrolizumab, the patient had a clinical and radiographic response. All of his skin lesions resolved except one, which continues to shrink clinically.
Patient 2 initially underwent resection, chemotherapy and radiation therapy. After new lesions developed, he had a second resection, neck dissection, and received treatment with nivolumab. The patient then developed new lesions and new retroperitoneal disease, for which he opted to receive nivolumab and radiation.
Pembrolizumab plus TVEC was started when the patient developed a new right-sided neck mass. Magnetic resonance imaging of the brain when he subsequently experienced neurologic symptoms showed a left occipital lesion, for which the patient underwent gamma knife radiosurgery. He has continued to receive pembrolizumab with no disease progression, and recent imaging shows a complete radiographic response.
Advertisement
Prior to the combination therapy, “these two patients really had no options left,” Dr. Gastman says. “Their disease was very refractory to everything before. Now it appears that they are cured.” He notes that the intralesional TVEC injections produce few side effects. “Fevers and chills are common, but that’s pretty much it,” he says.
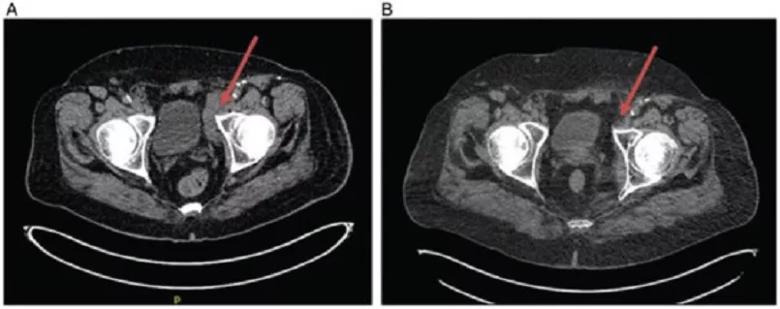
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/2a466168-a196-4be8-ac5b-5fdd158bf49e/19-CNR-4146-MMC-case-report-805x319-1_jpg)
Patient 1 imaging: (A) February 2017 demonstrating bulky inguinal adenopathy as indicated by arrow. (B) February 2018 demonstrating resolution of adenopathy as indicated by arrow. This reduction in adenopathy was observed despite talimogene laherparepvec injections only at site of lesions in lower extremity and not directly into adenopathy.
The investigational nature of the combination therapy posed a significant financial issue. “TVEC is not yet approved for MCC, so we had to make a case to the patients’ insurance companies for reimbursement,” says Dr. Gastman. “But because this disease is so rare and there were no treatments, they gave us approval.”
Dr. Gastman notes that there are currently only four papers in the world medical literature describing the use of TVEC to treat MCC. Two of them — including the Cleveland Clinic report — are on use of TVEC in combination with pembrolizumab. The other case report on the TVEC/pembrolizumab combination, published in 2018, was by investigators from the University of Southern California.
“No one has treated two cases with this specific combination therapy before,” says Dr. Gastman. “The previous report was on a single patient who didn’t have the same level of response as our patients.”
Advertisement
Dr. Gastman says it remains to be determined whether TVEC plus pembrolizumab is the optimal immunotherapy combination for refractory MCC, but no clinical trials of it are currently planned. The Cleveland Clinic Cancer Center investigators hope that, given the rarity of the disease, the positive outcomes of their two cases plus the one from the University of Southern California will be sufficient for the FDA to grant approval for use of TVEC plus pembrolizumab in patients with multi-drug-refractory MCC.
“Merkel cell cancer is deadly, but as with melanoma, the number of potential therapeutic options for it is growing,” says Dr. Gastman. “These diseases produce visible tumors and even if they are unresectable, injectable agents may hold promise.”
A key challenge for researchers studying MCC is finding enough eligible patients to enroll in clinical trials. “We have trials for patients with stage 1, 2 and 3 MCC, immunotherapy-naïve MCC and immunotherapy-refractory disease,” he says. “We may soon be launching a trial for patients with extreme refractory MCC. But many individuals with the disease are over 90 years old, and age alone is a barrier to their participation. Overall health status also can be an issue in these patients.”
One currently accruing MCC trial for which Dr. Gastman is the principal investigator is ECOG-ACRIN 6174. It is a Phase III study comparing pembrolizumab with standard of care observation for surgically resected MCC. He encourages physicians who have patients they think may be eligible to contact him.
Advertisement
“We would definitely be interested in taking care of patients with MCC,” he says. “For the first time in human history, we have therapies that make refractory MCC less common. There are minimal treatment options for it, but the good news is that there are people like the teams at Cleveland Clinic who are interested in finding cures for the small percentage of patients with a rare cancer.”
Feature image: Photomicrograph of Merkel cell carcinoma.
Advertisement
Combination therapy improves outcomes, but lobular patients still do worse overall than ductal counterparts
Bringing empathy and evidence-based practice to addiction medicine
Supplemental screening for dense breasts
Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors
Early results show strong clinical benefit rates
The shifting role of cell therapy and steroids in the relapsed/refractory setting
Radiation therapy helped shrink hand nodules and improve functionality
Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches