Model is the first continuous risk score for liver cancer
Hepatocellular carcinoma is the fifth leading cancer diagnosis worldwide and the fastest rising cause of cancer-related deaths in the United States. For patients who are not candidates for hepatectomy, the only option is a liver transplant. Given the limited number of donated organs, choosing the most appropriate candidates is critical.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
“When liver cancer recurs after liver transplantation the outcome is dismal, and because organs are limited we need to allocate them to patients who can benefit most” says Federico Aucejo, MD, Director, Liver Cancer Program, Cleveland Clinic’s Digestive Disease & Surgery Institute.
For two decades, the Milan criteria have been the gold standard for selecting liver cancer patients as transplant candidates. However, the Milan criteria have significant drawbacks: They only assess tumor morphology, such as size and number of tumors, and don’t consider tumor biology, patient’s overall health or changes in oncological risk over time.
“The Milan criteria are a binary system: the patient either meets the criteria or not,” says Kazunari Sasaki, MD, a clinical fellow in liver transplant surgery. “They are not accurate in predicting outcomes. There are patients who don’t meet the criteria but do well after transplantation based on tumor biology.”
With a goal of overcoming the limitations of the Milan criteria, Drs. Aucejo and Sasaki and colleagues from Cleveland Clinic’s Digestive Disease & Surgery Institute developed a new scoring system for assessment of overall survival following liver transplantation for liver cancer: HALT-HCC (Hazard Associated with Liver Transplantation for Hepatocellular Carcinoma).
HALT-HCC includes the following criteria:
Advertisement
To assess HALT-HCC, the research team conducted a retrospective cohort analysis using data from 420 patients with liver cancer who underwent liver transplantation at Cleveland Clinic between January 2002 and November 2014. Using multivariate Cox regression analysis, a risk equation was generated based on the association of HALT-HCC variables with overall survival. In the cohort, prognosis worsened with increasing HALT-HCC score (Figure 1).
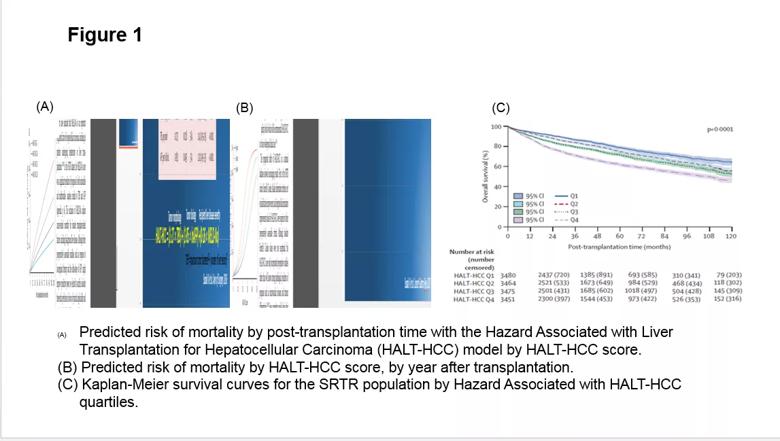
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a0224262-9f23-4c3a-b466-ebcad24e2672/Figure-1_png)
Further risk assessment and validation was done using nationwide data for the same time period from the Scientific Registry of Transplant Recipients (SRTR) based on a cohort of 13,717 patients. Patients within and outside the Milan criteria showed a similar risk of death when stratified by the HALT-HCC score (Figure 2).
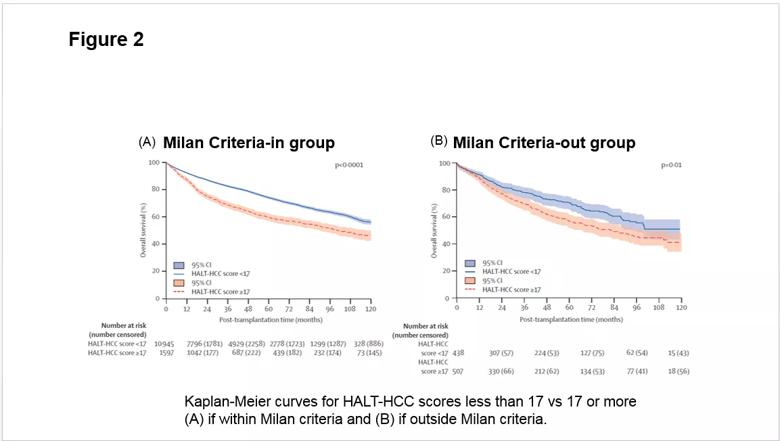
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/6806610d-2067-4263-a5a1-e3ae936dc083/Figure-2_png)
Overall, the HALT-HCC model outperformed traditional selection criteria on both datasets on a variety of statistical metrics (Table 1). The study findings appear in The Lancet Gastroenterology & Hepatology.
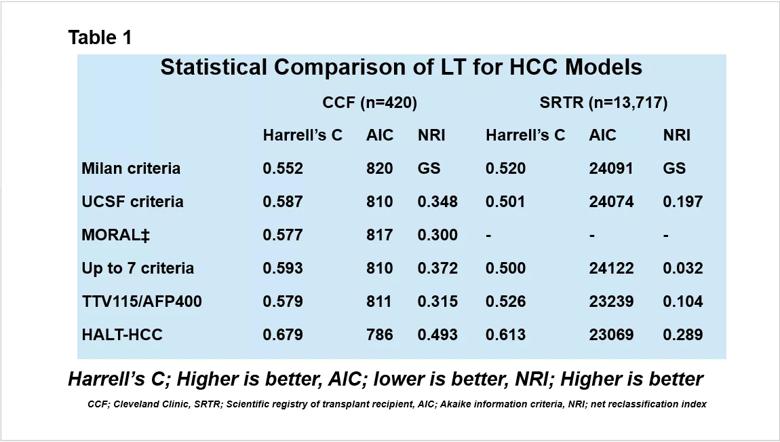
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d25a44cf-4198-43c6-a747-6f79f5f7507e/Table-1_png)
“The findings show that HALT-HCC criteria provided more risk stratification compared to other existing criteria. It can predict more accurately the risk of dying after transplantation. In that way, it is clearly superior,” says Dr. Aucejo.
This study established, for the first time in the field, the concept of a continuous multivariable risk measure for liver transplantation in patients with hepatocellular carcinoma. A major advantage of this model is that it can incorporate changes in the patient’s oncological risk over time and therefore predict who has the best chance of living longer after transplantation. HALT-HCC could also be a metric to assess response to loco-regional therapy. For example, a patient whose tumor has a good response to loco-regional treatment could move higher on the list for organ donation.
Advertisement
Since the first two studies were completed, the research team has continued its assessment and validation of HALT-HCC. They have recently completed a multicenter study in the U.S. that involved evaluating patients at various time points and have submitted a paper for publication. Currently in progress is a major international study with more than 4,000 patients from centers in the U.S., Canada, Europe and Asia.
“Our most recent studies have shown that HALT-HCC is able to predict the risk of recurrence, not just overall survival,” says Dr. Sasaki.
The final steps to acceptance of this new selection criteria are advanced statistical simulation modeling adapting to regional patient waitlist times and evaluation through large prospective studies.
“Based on the results so far, HALT-HCC is practical, easy to implement and outperformed other selection criteria. It has the potential to become a new clinical tool that can lead to more efficient use of donated organs,” says Dr. Aucejo.
Advertisement
Advertisement

Benefits of neoadjuvant immunotherapy reflect emerging standard of care

Multidisciplinary framework ensures safe weight loss, prevents sarcopenia and enhances adherence

Study reveals key differences between antibiotics, but treatment decisions should still consider patient factors

Key points highlight the critical role of surveillance, as well as opportunities for further advancement in genetic counseling

Potentially cost-effective addition to standard GERD management in post-transplant patients

Findings could help clinicians make more informed decisions about medication recommendations

Insights from Dr. de Buck on his background, colorectal surgery and the future of IBD care

Retrospective analysis looks at data from more than 5000 patients across 40 years