4-D imaging informs complex aortic valve repair in adult and pediatric patients
In the operating room, Hani Najm, MD, Chair of Pediatric and Congenital Heart Surgery at Cleveland Clinic Children’s, has typically relied on his vast experience to repair a defective aortic valve he has only seen on two-dimensional echocardiography.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
These days, it’s a different story. He knows every aspect of the valve in intimate detail and has determined his repair approach well in advance of the surgery. It’s an extraordinary advantage only made possible by the addition of Justin Tretter, MD, to the staff of the Congenital Valve Procedural Planning Center.
A rare find, Dr. Tretter is a pediatric cardiologist with fellowship training in advanced noninvasive imaging and additional subspecialty training in cardiac morphology.
“He is one of the rare anatomists of the heart,” says Dr. Najm. “His images provide information on every aspect of a valve and its root, as well as on the relationship of structures lying beneath what is visible to me as a surgeon. Knowing in advance where the problems are located gives me confidence that when I fix them, there is high likelihood the repair will work.”
Together, Drs. Najm and Tretter serve as Co-Directors of the Congenital Valve Procedural Planning Center at Cleveland Clinic Children’s.
An early fascination with the cardiovascular system and a love of children steered Dr. Tretter into pediatric cardiology. But it was a chance encounter with world-renowned cardiac anatomist Robert H. Anderson, MD, PhD, that took his career path in a unique direction. “We shared a passion for understanding the detailed structure of both the normal heart and those with congenital heart defects,” he says.
Under Dr. Anderson’s direction, he learned to study autopsy specimens of both normal and diseased hearts to understand the details of anatomy that are relevant to surgeons and interventionalists. “My goal was to learn how to image these details and use the information to bring personalized surgical planning to the next level,” he says. “To accomplish this, I wanted to work with the best congenital heart surgeon in the world, and that’s Hani Najm.”
Advertisement
Obtaining the level of detail he sought required three-dimensional (3-D) imaging. Dr. Tretter often uses multiple imaging modalities to comprehensively evaluate their patients, including echocardiography (Figure 1) and often a cardiac MRI, the advantage of which is “a very accurate assessment of heart function and the ability to quantify the extent of leakage or obstruction.” However, he found a CT was best-suited for the detailed 3-D imaging needed to personalize aortic valve surgical planning, which requires detailed measurements to understand the geometry of this complex structure. The newer scanners at Cleveland Clinic produce detailed 3-D images—four-dimensional (4-D) if movement is included (Video 1)—with minimal radiation.
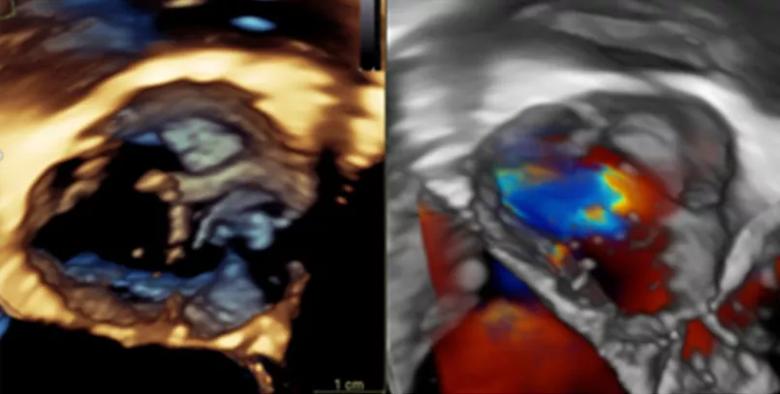
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/fb0b613b-2403-4f27-a5a8-f5c0063fe4b9/22-CHP-3164082-CQD-Tretter-Najm-CCCValvePlanCtr805x407-1_jpg)
Figure 1. Drs. Tretter and Najm use standard echocardiography imaging to assess the aortic valve, including 3-D echocardiography, as shown here. However, this assessment can be limited depending on the patient’s age and body size. Video 1. An example of a 4-D CT reconstruction of a bicuspid aortic valve.
The physicians review detailed CT images and measurements of the aortic root structure, which includes not only the aortic valve leaflets, but also the sinuses and fibrous interleaflet triangles, found between the leaflet attachments. This helps to identify the unique characteristics of a valve and its defect.
With 4-D imaging, he is able to mathematically quantify and illustrate in exquisite detail how a bicuspid or unicuspid aortic valve functions (Figure 2, Video 2). “Historically, surgeons rely on 2-D ultrasound images, occasionally on 3-D ultrasound images, which may be limited, and largely rely on their small window in the operating room to assess the valve when the heart is stopped and blood removed,” he says. “By adding our 4-D CT assessment preoperatively, we provide a detailed blueprint of what the problem is and are able to develop a detailed plan to fix it before stepping foot into the operating room.”
Advertisement
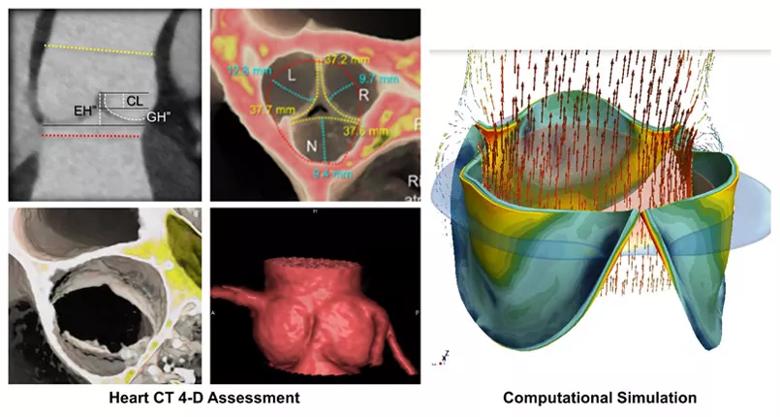
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ad13acc2-950a-4d90-bb6f-9f4ae1dac831/22-CHP-3164082-CQD-Tretter-Najm-CCCValvePlanCtr805x407-2_jpg)
Figure 2. Once the cardiac CT is obtained, Dr. Tretter uses the 2-D images (upper left-hand panel) to make detailed measurements of the structures of the aortic root and aortic valve. He then creates 3-D and 4D reconstructions from the cardiac CT to help visualize the detailed structure of the aortic valve to Dr. Najm, where they are able to decide the best repair or reconstruction strategy. They are working with bioengineers to then simulate the repair and predict the durability of the repair over time. Video 2. This 3-D and 4-D CT reconstruction demonstrate the ability of Dr. Tretter to demonstrate the measurements of the aortic root and its aortic valve in exquisite detail and display this quantitative assessment to Dr. Najm, cutting in multiple planes of the heart.
The images can be sliced at any level to give Dr. Najm a look at structures otherwise invisible to him during surgery. As he proposes ways to restore the valve’s natural shape, he is able to visualize how it will function.
“When I ask Dr. Tretter to cut his 4-D CT scan images here or there, I can see how it will work and what will happen if I change my approach,” he says. (Video 2)
After selecting the optimal plan for reconstructing the valve and root complex, he creates a plan to use as a blueprint for the operation. “There is no ambiguity, no surprises,” he says.
Working with advanced imaging to plan a repair strategy minimizes the number of valve procedures the patient will need in their lifetime.
“Aortic valve repair has not always been effective. Knowing in advance where the problems are, I am encouraged, because I know there is a high likelihood the repair will work,” says Dr. Najm.
Advertisement
While Dr. Tretter is supplying 4-D CT images to guide valve repair, he is investigating other uses of imaging to improve outcomes of surgical and interventional procedures. Two of these efforts include fine-tuning root replacements in asymmetric, severely regurgitant aortic valves and identifying the location of the other critical structures the surgeon cannot see, such as the conduction system.
“We are working out ways to predict where the conduction system is positioned in relation to the valve, so the surgeon can avoid causing collateral damage,” Dr. Tretter explains.
They are also actively using advanced imaging to evaluate patients who require surgery for aortic valve problems following surgery for tetralogy of Fallot, truncus arteriosis, transposition of the great arteries, ventricular-septal defects and other heart problems that can result in aortic valve disease. “We are developing an algorithm for imaging and the best surgical repair or reconstruction strategies using 4-D imaging and computational modeling,” says Dr. Tretter (Video 2).
Efforts to improve the success of congenital and pediatric aortic valve surgery are supported by a heart form-to-function lab. Currently, Dr. Tretter is working with a bioengineer from KTH Royal Institute of Technology, Dr. Elias Sundström, to create models of the aortic valve and root from detailed measurements he provides. “By manipulating the model, we can simulate the surgery and look at the resulting hemodynamics. A few years down the road, we will know if we can use these models to predict long-term success,” he says.
Advertisement
“I’ve been fortunate to develop effective valve-repair methods based on my huge experience, but without advanced imaging, we don’t know which repair techniques are best,” says Dr. Najm. “As we continue to publish our findings based on personalized imaging, we look forward to recommending different techniques that even less-experienced surgeons can do.”

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ee6b2ead-dfc4-41ee-9b6e-15cfd98a4d40/22-CHP-3431758-Pediatric-2022-Year-in-review-footer-2_jpg)
Advertisement

Join us in New York City Dec. 5-6

Study examines data and clinical implications for performing Ross procedures in infancy versus later in life
Choice between smaller or larger prosthesis is a tradeoff between leak and pacemaker risks
Study offers guidance on an increasingly common presentation

Cardiac imaging substudy is the latest paper originating from the VANISH trial

Indication, timing and options for surgical intervention

30-year study of Cleveland Clinic experience shows clear improvement from year 2000 onward

Large, multinational trial finds no significant difference in vigorous versus nonvigorous exercise