Unique skin changes can occur after infection or vaccine
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d2d7c4be-50d5-4efe-9c32-f81923eff726/coronavirus-GettyImages-1200706447-jpg)
Coronavirus
By Samantha Polly, MD, Inga M. Muser, MD and Anthony Fernandez, MD, PhD
This article is reprinted (without references) from the Cleveland Clinic Journal of Medicine (2023;90[1]:43-52). The fully referenced original article is available here.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
As the impact of the COVID-19 pandemic has extended across the globe, cutaneous presentations deserve specific attention in both adult and pediatric patient populations. The development and dissemination of COVID-19 vaccines also have generated notable cutaneous findings, and studies have noted skin phenotype as a factor in cutaneous COVID-19 manifestations. Recognition of these reactions and their implications is beneficial to clinicians in shaping patient counseling and anticipatory guidance.

Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7ae06ea5-ad84-4ccb-b136-dc7d94cb6944/23-PSX-3535263-CQD-Hero-650x450-1-jpg)
An 11-year-old received vaccines in the right arm for tetanus, diphtheria, acellular pertussis and meningitis. In the left arm, he received an mRNA COVID-19 vaccine and the human papillomavirus vaccine. Within minutes, he developed a large, pruritic, erythematous and edematous plaque on his left arm and subsequent vesiculation. Over the next two days, this progressed to a large, painful ulceration. He had no systemic symptoms. The lesions eventually healed.
The reported prevalence of cutaneous manifestations ranges between 0.25% and 8.1% in pediatric COVID-19 cases, with studies suggesting that the face is the most commonly affected site. Review articles have detailed similarities in cutaneous findings and their implications between adult and pediatric patients.
However, unique manifestations have been described in children, including erythema multiforme, acute hemorrhagic edema of infancy, papular acrodermatitis of childhood, and various skin changes in multisystem inflammatory syndrome in children (MIS-C) that we will detail in this article.
An early review article found that cutaneous involvement was described prior to other systemic symptoms in 77.9% of pediatric COVID-19 cases and simultaneously in 13.2% of cases. Additionally, a cohort study of more than 12,000 children noted that the prevalence of fever in conjunction with cutaneous lesions was lower in adolescents when compared with younger children and infants. Their analyses also suggested that hospitalized COVID-19 pediatric patients more frequently had rash, urticaria and conjunctivitis at the time of presentation compared with nonhospitalized patients, although specific incidence rates and comparative statistics were not reported.
Advertisement
Although there is overlap in the cutaneous manifestations between adult and pediatric populations, the most notable cutaneous abnormalities in pediatric COVID-19 patients relate to MIS-C. Reported findings in this syndrome include a nonexudative conjunctivitis, polymorphic rash, oral mucositis, hand and foot anomalies, and perineal and facial desquamation.
These manifestations suggest that MIS-C shares many similarities with Kawasaki disease. However, children with MIS-C tend to be older, with higher rates of gastrointestinal symptoms, myocarditis and shock than in classic Kawasaki disease. Mucocutaneous manifestations are important clues to the diagnosis of MIS-C,although not significantly associated with overall disease severity, and in some studies have been associated with lower rates of intensive care unit admission, shock, and requirement for invasive mechanical ventilation.
As of September 2021, the U.S. Centers for Disease Control and Prevention (CDC) reported that rates of COVID-19 infections were 1.1 and 1.5 times higher in Black and Hispanic populations, respectively, compared with white peers. Notably, these differences increased when rates of hospitalization and death were considered.
Despite this, there has been a relative dearth of published information describing these findings. Interestingly, three studies based on race and ethnicity found that COVID-19–specific cutaneous manifestations, including chilblain-like lesions, were uncommon in patients with darker skin phenotypes. As these studies were relatively limited in size, it is not yet clear whether these observations reflect the subtleties of appreciating inflammation in darker skin tones or true variations in presentation.
Advertisement
Additionally, multiple small retrospective studies found disproportionate rates of telogen effluvium in patients with darker skin tones during the COVID-19 pandemic, and the presence of medical comorbidities has been described as a risk factor. However, larger prospective studies are needed to clarify this association.
Furthermore, self-reported rates of scalp erythema and scaling were significantly higher in non-white Brazilian patients with confirmed COVID-19 infection. Our institutional experience suggests cutaneous abnormalities seen in patients with darker phenotypes may be somewhat more variable compared with those described and seen in white patients.
Although more studies are needed to better characterize the skin manifestations of COVID-19 in patients with darker skin, and to investigate potential prognostic implications, a recent review highlighted clinical clues for identifying predominant cutaneous findings in patients with darker skin tones and emphasized the importance of palpation when considering diagnoses of urticaria, morbilliform eruptions, or even chilblain-like lesions, as the associated erythema may be more difficult to appreciate.
Additionally, hyperpigmentation was noted to provide insight into previous skin inflammation and may be concerning to affected patients.
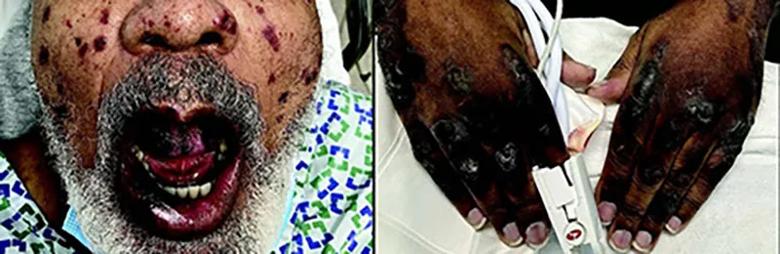
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/a93d4830-91d6-496c-9812-7b013e24b05a/23-PSX-3535263-CQD-Inset2-800x260-1_jpg)
A 74-year-old man, fully vaccinated against COVID-19 and with a remote history of cutaneous leukocytoclastic vasculitis, was seen in the emergency room after developing new purpuric patches, plaques, and bullae on the face, oral mucosa, trunk and extremities. Testing confirmed acute breakthrough COVID-19 infection. He was discharged in stable condition after a 22-day hospital stay.
Vast clinical trial data noted that self-limited, nonspecific, acute, local reactions were the most commonly described cutaneous findings following vaccination with a messenger RNA (mRNA) vaccine. However, a study of 414 cutaneous reactions to mRNA COVID-19 vaccines noted the most frequently reported reactions were delayed large, confluent, local reactions involving the lateral upper arm and deltoid at the injection site, often referred to as “COVID arm.”
Advertisement
International registry data suggest delayed cutaneous reactions are more commonly seen with the Moderna mRNA vaccine and in female patients, presented on average one week after the first vaccine dose, with no reports of severe adverse events in patients who went on to receive a second dose. The reaction is often associated with mild tenderness and pruritus, and less commonly with concomitant fever and malaise, resolving within one to two weeks without treatment.
In our experience, nonconfluent rashes, rashes involving the lateral arm, or rashes involving other anatomic locations close to the injection site may also occur. Notably, when looking at all cutaneous reactions in the above-mentioned study, 43% of patients who initially had a cutaneous reaction developed another cutaneous reaction after the second dose.
Other commonly reported cutaneous manifestations include findings associated with COVID-19 infection, such as functional angiopathies, urticarial eruptions, and morbilliform rashes. Another important adverse event to be aware of is herpes reactivation, which was reported in 13.8% of cutaneous reactions from a cohort of 405 cases. A review of 40 cases of herpes reactivation found that none of the patients had a repeat viral flare after the second vaccination dose.
The connection between reactogenicity and immunogenicity of the mRNA vaccines continues to be explored. Recent studies have suggested that systemic adverse effects are correlated with increased antibody production. However, others have found that the presence and severity of local and systemic adverse reactions are not reliable indicators of a humoral response, specifically when adjustments are made for age and sex that have been independently associated with increased antibody production.
Advertisement
As the number of vaccinated patients continues to climb and increasingly available metrics of immune response are developed, the relationship between cutaneous reactions and immunogenicity may become clearer.
Owing to more recent approval of the Pfizer-BioNTech COVID-19 vaccine for pediatric patients, data regarding cutaneous reactions in this population are limited. However, clinical study data noted that rash was seen in 0.3% of children ages 5 to 11; redness and swelling at the injection site were reported in less than 20% of patients.
As increased numbers of pediatric patients undergo vaccination, clinicians should be familiar with commonly seen reactions as well as the potential for more severe presentations, which may relate to a more intense immune response in younger individuals. Additionally, a recent nationwide analysis of French adolescents ages 12 to 18 suggests that COVID-19 mRNA vaccination could be associated with a lower incidence of MIS-C, although data regarding younger patients are not yet available.
Although the Johnson & Johnson adenoviral vector vaccine appears to have relatively few dermatologic side effects, with clinical trial data and safety analyses reporting only local adverse reactions and urticaria, rare reports of vaccine-induced immune thrombotic thrombocytopenia have garnered significant attention. Patients have demonstrated concomitant petechiae, suggesting their presence may be a clue to this reaction.
Although only a few cases describing vaccine-related cutaneous manifestations of immune thrombotic thrombocytopenia have been reported to date, affected patients are often critically ill, and subsequent deaths have been reported. Thus, any cutaneous manifestation that provides insight to this diagnosis could be valuable.
Other cutaneous reactions have also been reported in association with the Johnson & Johnson COVID-19 adenoviral vector vaccine.
Cutaneous findings of COVID-19 infection in pediatric patients appear to overlap those in adult patients. Although the constellation of cutaneous findings in MIS-C can aid in diagnosis, mucocutaneous involvement is not correlated with more severe disease.
While there are reports of fewer cutaneous findings in COVID-19 infection in non-White patients, palpation may be helpful in appreciating subtle inflammation not readily apparent on visual examination. Fortunately, the vast majority of cutaneous mRNA vaccine reactions are short-lived, associated with only minimal or mild symptoms, and in some cases represent molecular mimicry causing rashes similar to those seen with COVID-19 infection.
Given the limited number of available studies and variable strength of current data, future research is warranted to more definitively characterize cutaneous manifestations of COVID-19 in pediatric and non-White patients, in addition to cutaneous manifestation following COVID-19 vaccines.
Advertisement
Dynamic modeling improves the accuracy of outcome predictions for ICU patients
A review of IDSA and NIH guidelines
Study sheds light on how clinicians addressed their patients’ pain and insomnia during the pandemic
Patients report improved sense of smell and taste
Clinicians who are accustomed to uncertainty can do well by patients
Will enable patients with long COVID to enroll in national clinical trials
Cleveland Clinic analysis suggests that obtaining care for the virus might reveal a previously undiagnosed condition
Team collaborates to explore effects of genetic mutations to proteins OAS and RNase L