Successful preservation, evaluation and transplant of orphan livers could mark a major advance in the field
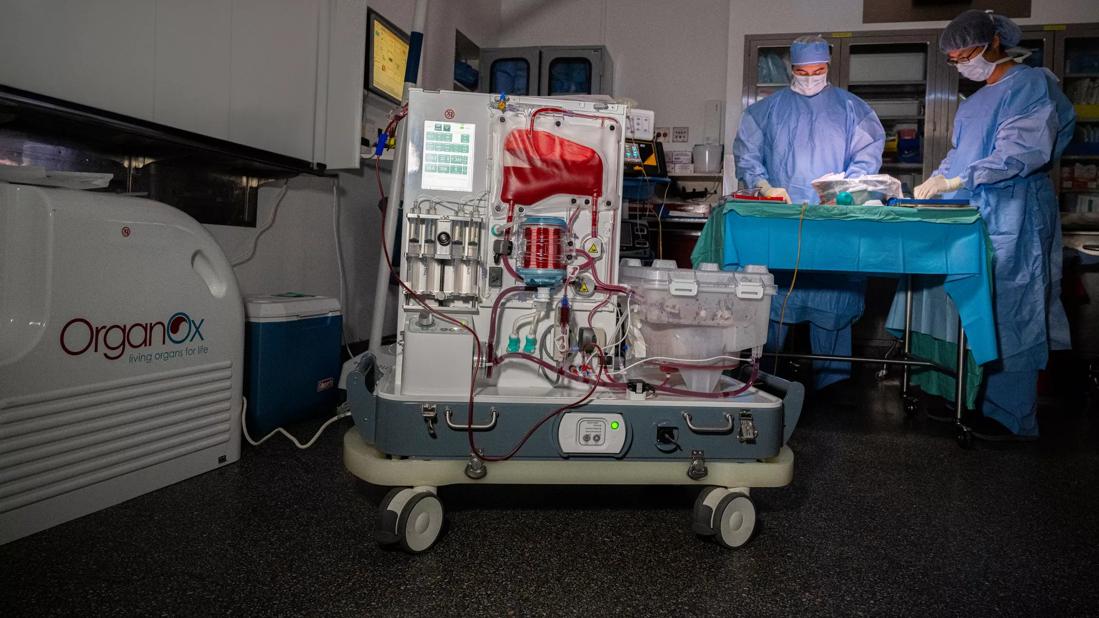
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8138033e-ff27-4b0d-97f5-18bc7370ddd5/Liver-transplant)
Perfusion machine
In a notable clinical trial result, a Cleveland Clinic-developed normothermic perfusion machine has enabled surgeons to assess and successfully transplant 15 of 21 “orphan” livers — 72% — that otherwise would have been discarded as unsuitable, the research team responsible for the device reports.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Because normothermic machine perfusion (NMP) maintains the donor liver in a metabolically active state, unlike traditional cold organ preservation, it allows the transplant team to evaluate the organ’s functionality prior to transplant and to potentially modify it by infusing nutrients and medications. The highly promising outcomes utilizing orphan livers suggest that organ viability standards should be revisited, the researchers say.
Their findings may herald a new era in transplantation in which livers previously deemed unusable are rejuvenated ex-situ and made available to alleviate severe donor organ shortages, reduce transplant candidates’ deaths while on waiting lists, and expand indications for transplantation. The Cleveland Clinic researchers estimate that NMP could increase the annual number of U.S. liver transplants by 14%, allowing an additional 1,200 patients per year to receive lifesaving organs.
The device also reduces the risk of reperfusion injury and significantly extends the interval that a donor organ can remain viable outside a patient’s body. That could make the logistics for longer-distance transports for transplantation more feasible and buy time to recondition even severely injured organs.
“Very few things in medicine have the ability to improve conditions by a dramatic amount,” says liver transplant surgeon Cristiano Quintini, MD, the newly appointed Chair of Cleveland Clinic Abu Dhabi’s Digestive Disease Institute and leader of the team developing and testing the NMP device. “This device is one of those things. We have a technology that I know can save lives. I would use it for every transplant patient if I could.”
Advertisement
The normothermic perfusion machine was developed in-house, using a combination of off-the-shelf parts adapted from cardiopulmonary bypass equipment and custom-built components. Dr. Quintini and his team have utilized the machine in 70 liver transplants over four years as part of an ongoing multiphase clinical trial. Machine perfusion, along with other innovations such as laparoscopic living donor liver transplantation, have helped Cleveland Clinic’s liver transplant program become a leader in the field.
The U.S. Food and Drug Administration currently considers Cleveland Clinic’s NMP device and other machine preservation devices experimental and very high risk. Additional research will be needed to secure regulatory approval, and subsequent commercialization would require substantial capital investment.
“Our goal is to continue to contribute to the body of knowledge about this technology,” Dr. Quintini says.
Ex-situ machine perfusion to preserve organs for transplant was explored in the early years of the liver transplantation, in the 1960s and ‘70s. The lack of success using hypothermic perfusate in those initial experiments and the simplicity and relative efficacy of static cold storage (SCS) have allowed the latter to remain the predominant means of ex-situ liver preservation.
Prolonged cold ischemia time during SCS can cause mitochondrial injury, resulting in ischemic cholangiopathy upon reperfusion and potential post-transplant graft failure. High-risk extended criteria donor livers — suboptimal organs such as macrosteatotic grafts or those from elderly donors, whose use is increasingly necessary in an attempt to meet transplantation demands — are more vulnerable to reperfusion injuries associated with SCS. SCS also precludes the ability to evaluate bile production and other active metabolic indicators of graft quality.
Advertisement
Although a variety of individual biomarkers and other measures of donor liver functionality exist, there is no standardized, comprehensive, evidence-based methodology to reliably determine organ viability.
“Every liver transplant surgeon has experienced the exceptionally traumatic events that accompany a nonfunctional organ after reperfusion,” says Dr. Quintini, who directed Cleveland Clinic’s liver transplant program prior to moving to Cleveland Clinic Abu Dhabi. “It’s a tragedy for the patient and for the transplant team.”
Therefore, “when we are in doubt about whether an organ will function after transplant, we choose the safest option, which is not to transplant,” he says. “Currently, 15% to 20% of the livers procured annually for transplant in the United States are not used for fear they may not work. Many times we leave organs behind that actually could be viable. A proportion of organs that we are discarding could diminish or even eliminate the mortality of patients on transplant waiting lists.”
In addition to improved preservation, machine perfusion creates the potential for organ viability testing and rehabilitative treatment prior to implantation. Researchers at various institutions are evaluating both hypothermic and normothermic approaches for liver transplantation.
Hypothermic machine perfusion (HMP) has been used since the 1990s to preserve kidneys for transplant, with better outcomes than SCS. In 2010, Columbia University Medical Center researchers reported the first successful transplantation of 20 human livers preserved using HMP. The researchers determined that HMP is safe, may improve graft function and reduces biomarkers indicative of liver preservation injury.
Advertisement
At hypothermic temperatures (between 4° and 10°C), organ metabolism is reduced by 90%, precluding pretransplant graft assessment of functions such as bile production, glucose consumption and lactic acid clearance. Machine perfusion at normothermic temperatures (≥35°C) preserves the liver in a fully functioning metabolic state, with oxygenation achieved by utilizing a perfusate containing red blood cells.
In 2013, researchers at the University of Oxford in the United Kingdom carried out the first series of human liver transplants using a NMP device, producing data on proof of concept, safety and logistics of the approach. Various clinical trials during the next decade have verified NMP’s ability to reduce preservation-related graft injuries and to improve the quality of marginal organs and allow them to be utilized in transplants. But there is no conclusive clinical data showing NMP’s superiority to HMP or SCS in terms of post-transplant complications and graft and patient survival.
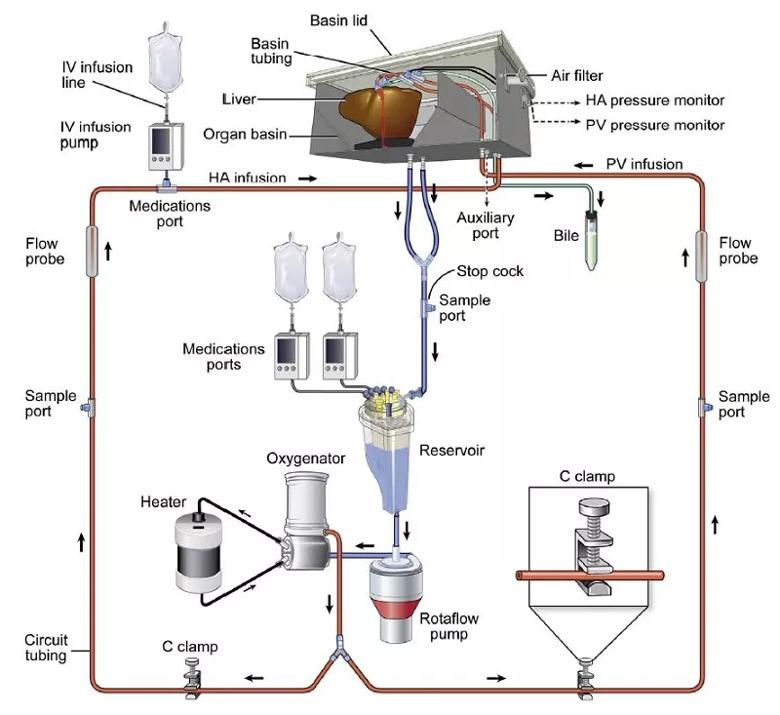
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/ffe0d1b3-2346-4cfe-8442-4feb3fe551ec/22-DDI-2714701-Normothermic-machine-perfusion-inset-800x550-1_jpg)
Figure 1. Diagram of Cleveland Clinic’s normothermic machine perfusion device. The liver perfusion circuit consists of a centrifugal pump, a venous hard-shell reservoir, a heater, an oxygenator and an institutionally developed organ receptacle. The circuit has a bifurcation between pump and receptacle and C-clamps on the tubing to portal infusion. Reprinted from Quintini C, Del Prete L, Simioni A, Del Angel L, Diago Uso T, D’Amico G, Hashimoto K, Aucejo F, Fujiki M, Eghtesad B, Sasaki K, Kwon CHD, Cywinski J, Bennett A, Bilancini M, Miller C, Liu Q. Transplantation of Declined Livers after Normothermic Perfusion. Surgery. 2022 Mar;171(3):747-756. Copyright 2022. Used with permission from Elsevier.
Advertisement
Dr. Quintini had followed the progress of the Oxford Group in the early 2000s as they tested the NMP concept in animal models. “I was convinced that normothermic perfusion was the future of liver transplantation,” he says. Working with Research Associate Qiang Liu, MD, Heart, Vascular & Thoracic Institute Director of Perfusion Services Patrick Grady, Engineering Technician John Etterling and others, Dr. Quintini led development of Cleveland Clinic’s NMP device and gained the institutional and governmental regulatory approvals for animal and human testing.
In 2015, the Food and Drug Administration OK’d Cleveland Clinic’s plan to conduct the first U.S. human clinical trial of transplantation of deceased-donor livers preserved using normothermic ex-situ machine perfusion.
The initial phase of that trial demonstrated that Cleveland Clinic’s noncommercial device — using fresh-frozen plasma-based perfusate for oxygenation, a first — was safe and effective over the course of 25 liver transplants, reducing the rate of early allograft dysfunction compared to a historical control group of liver transplant recipients.
Dr. Quintini and colleagues also showed that their innovative normothermic machine could dramatically prolong a liver’s viability ex-situ. In a boundary-pushing experiment using an organ declined by other transplant centers, the device sustained the liver’s metabolism, bile production and lactate clearance for 86 hours — more than three times the longest previously reported ex-situ maintenance of human liver metabolism. The organ was not used in a transplant. Post-experiment histology showed normal parenchyma, preserved sinusoidal architecture and viable hepatocytes.
“We don’t know the full potential of normothermic technology yet, but we think we eventually could achieve 10 times longer preservation time than the current technology,” Dr. Quintini says.
The extended preservation period made possible by NMP has proved beneficial during the COVID-19 pandemic. When a donor liver becomes available, the compatible recipient must travel to the hospital and undergo a COVID test with a negative result for the transplant to proceed — a process that can take several hours in addition to the period of time the organ is in transit. “If we didn’t have the normothermic perfusion device, the amount of ischemia a liver would experience [during the interval for COVID testing] would be prohibitive, even for an optimal organ,” Dr. Quintini says. “Having the pump gives us a 10- to 12-hour window we otherwise wouldn’t have. We’ve transplanted a couple of dozen patients during the COVID era and that would not be possible without machine perfusion.”
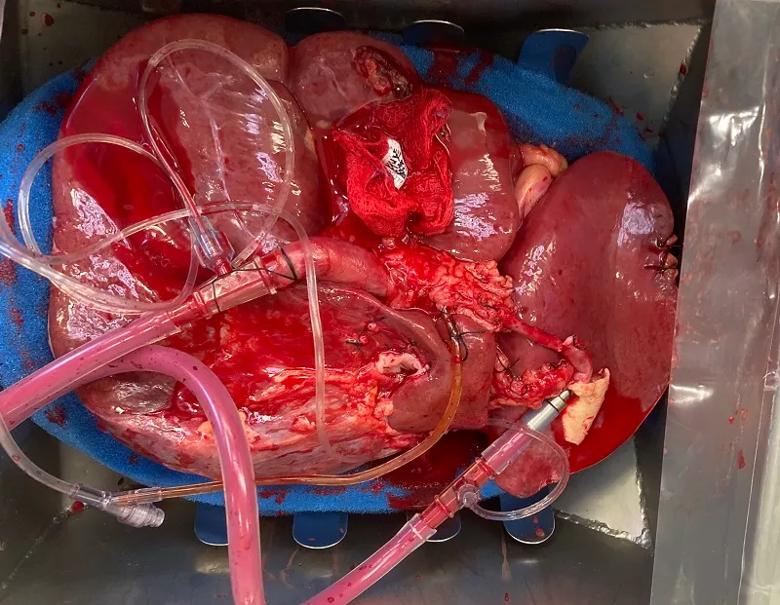
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8661fc1f-1800-4cbb-8502-d130c2c59558/22-DDI-2714701-Normothermic-machine-perfusion-inset-2-800x550-1_jpg)
A view looking downward into the normothermic perfusion machine’s organ basin shows cannulation on the donor liver’s portal vein, hepatic artery and bile duct. The tubing brings perfusate inflow to the liver and collects bile produced by the organ.
In the most recent phase of the ongoing NMP clinical trial, 21 livers designated for transplant but declined by other transplant centers between March 2020 and May 2021 were transported to Cleveland Clinic for enrollment in the study. All but one of the organs had been preserved using SCS prior to their arrival and connection to the normothermic perfusion device; a single liver procured at Cleveland Clinic was placed directly on the machine without cold preservation.
Perfusate samples were assessed hourly for levels of bilirubin, blood gases, electrolytes, lactate, glucose, lactate dehydrogenase and aspartate and alanine transaminase. Bile production and composition also were regularly monitored. The transplant team made a viability determination within six hours of the start of perfusion. To be considered for transplant, each organ had to meet at least two of four criteria:
Fifteen livers were deemed viable and subsequently transplanted. Time on NMP ranged from 3 hours 49 minutes to 10 hours 29 minutes. There were no technical problems, adverse events, intraoperative or major early postoperative complications, or cases of primary nonfunction after transplant. Seven livers had early allograft dysfunction with rapid recovery. One patient developed ischemic cholangiopathy four months post-transplant, was treated with biliary stents and is awaiting retransplant. All other patients had good liver function with follow-up times of 13 to 25 months.
The successful post-transplant functioning of the 15 livers preserved with NMP — despite exhibiting varying measures of viability, such as lactate clearance and glucose metabolism — led Dr. Quintini and his colleagues to conclude that current viability criteria need to be carefully expanded, and that it is time to take more risks regarding utilizing organs that are considered marginal by traditional standards.
While normothermic perfusion shows considerable promise in liver transplantation, it is unclear whether it is superior to hypothermic perfusion in terms of outcomes. “One of the advantages of our platform is that we can do both cold and warm perfusion,” Dr. Quintini says. “As part of our research, we are working on a study to see if hypothermic machine preservation followed by normothermic perfusion (as proposed by the University Medical Center Groningen group, combining warm and cold technologies) improves our ability to preserve and test livers. We’re not sold on warm versus cold perfusion and it is likely that in the future a tailored approach will be necessary for different types of livers. What we’re sold on is the overall potential of machine preservation.”
An additional question is whether initiating NMP immediately after extirpation of the donor liver is more advantageous than a period of SCS followed by machine perfusion, as has been the case with most Cleveland Clinic transplants and those of other programs testing the technology. Chinese researchers in 2020 reported having safely performed 28 ischemia-free liver transplants (IFLTs) utilizing NMP to procure, preserve and implant the organs without interruption of their blood supply. Their account did not attempt to compare outcomes of IFLTs versus initial SCS/subsequent NMP, but the authors concluded that IFLT might make graft viability assessment faster and easier.
NMP-aided IFLT “is a huge step toward utilization of every possible organ,” Dr. Quintini says. Cleveland Clinic’s NMP device is portable enough to be transported to the donor site to enable IFLT, although the logistics could be complex. “I suspect that when we have fully miniaturized and inexpensive devices, IFLT will become commonplace, but that will require considerably more investment in research and development.”
Machine perfusion-aided manipulation, rehabilitation and treatment of suboptimal livers and other donor organs is another aspirational goal. Delivering therapeutic agents — cellular, genetic or biological — via normothermic or hypothermic perfusate would directly target the donor organ while avoiding unwanted systemic effects that could be detrimental to the patient.
“You can manipulate the organ while it is on the device, doing things that you cannot do with a patient whose other organs and systems could potentially be injured by your intervention,” Dr. Quintini says. “With machine perfusion, you have an isolated system that you can use to change or improve the organ as needed prior to transplant.”
Potential therapies include prevention of acute rejection and ischemic reperfusion injury and modification to restore normal organ function or correct disease (such as “defatting” of steatotic donor livers). Experimental studies of perfusion-aided therapies in animal models and nontransplanted human organs to date have demonstrated proof of principle, safety and limited efficacy in some circumstances, but much more research is needed.
At the outset of Cleveland Clinic’s project to research and develop machine perfusion for liver transplantation, Dr. Quintini and his colleagues decided to build their own device to expedite the process, rather than partnering with a medical equipment manufacturer or other commercial entity. That resourcefulness has a long tradition at Cleveland Clinic, dating back to Dr. Willem Kolff’s work in the 1950s to develop a successful cardiopulmonary bypass machine and an artificial heart.
“This was a strategic decision we made six or seven years ago when there were no commercial devices that we could use in the United States,” Dr. Quintini says. “We wanted to be at the forefront of this technology because we believed it could save many lives. When you’re conducting a study sponsored by a commercial entity, you have to follow the rules they set forth. We felt that having our own device would allow us to have more flexibility on the studies. We were able to create a device that we could use the way we believed was appropriate and safe, and we could fine-tune our protocols as we learned more about the technology.”
Moving forward, a multicenter clinical trial will be needed to collect the data to support regulatory approval and commercialization of Cleveland Clinic’s NMP device.
“A study like that is complex and extremely expensive,” Dr. Quintini says. “I hope we can collaborate with a commercial partner who believes in our principles and our device, to get this technology into the community. It has huge potential. The more organs we can rescue and have available, including for patients who traditionally have not been considered good candidates for liver transplants, the more we can push the boundaries of transplantation.”
Advertisement
Benefits of neoadjuvant immunotherapy reflect emerging standard of care
Multidisciplinary framework ensures safe weight loss, prevents sarcopenia and enhances adherence
Study reveals key differences between antibiotics, but treatment decisions should still consider patient factors
Key points highlight the critical role of surveillance, as well as opportunities for further advancement in genetic counseling
Potentially cost-effective addition to standard GERD management in post-transplant patients
Findings could help clinicians make more informed decisions about medication recommendations
Insights from Dr. de Buck on his background, colorectal surgery and the future of IBD care
Retrospective analysis looks at data from more than 5000 patients across 40 years