Largest-to-date evaluation of germline mutations in this population demonstrates the value of genetics evaluation and genetic testing

Solid tumors account for half of cancer cases in children, adolescent and young adult (C-AYA) patients. The majority of these cases are assumed to result from germline variants rather than somatic alterations. However, little is known regarding the spectrum, frequency and implications of these germline variants.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
In this study, led by Charis Eng, MD, PhD, chair of Cleveland Clinic’s Genomic Medicine Institute, the researchers conducted the largest-to-date evaluation of germline mutations in C-AYA patients with solid tumors utilizing a combined dataset from Cleveland Clinic and St. Jude Children’s Research Hospital. Of the 1,507 patients analyzed, 12% carried germline pathogenic and/or likely pathogenic variants in known cancer-predisposing (KCPG) genes while an additional 61% had germline pathogenic variants in non-KCPG genes. The study was published in Nature Communications.
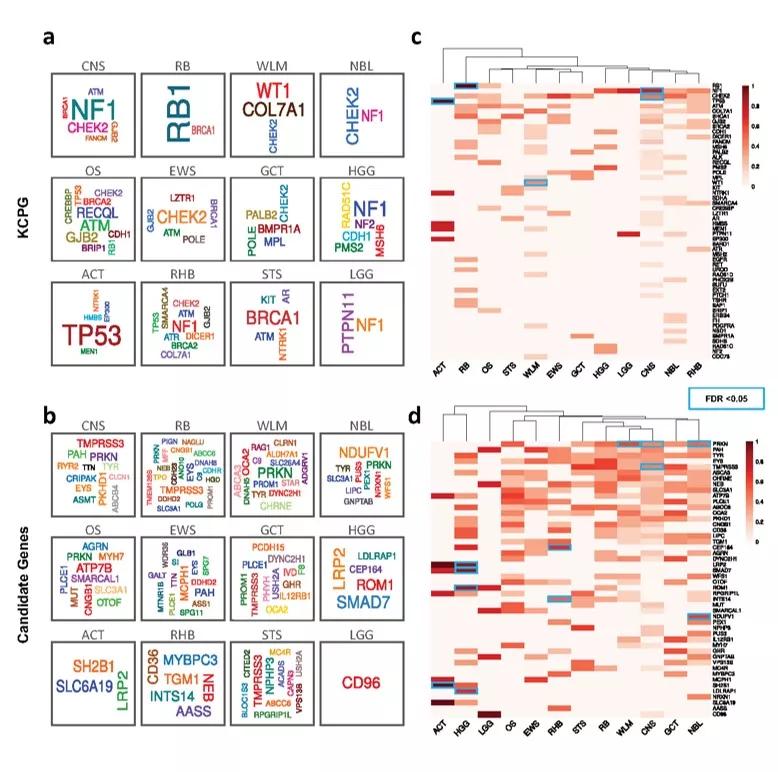
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/d1706e09-7853-4c3a-af2f-cedd6c415f2e/fig-3-for-web_jpg)
a, b Germline gene cloud signatures of the C-AYA patients with solid tumors based on their altered known cancer-predisposing genes (KCPG) (a) or candidate genes (b). The size of the genes is proportional to their pathogenic/likely pathogenic (P/LP) variant frequency in that tumor type, colors do not specify any meaning. c, d Heat maps of top altered KCPGs (c) or candidate genes (d). Two-sided Fisher´s Exact test implemented in R statistical software. P values were adjusted for multiple testing with Bonferroni correction considering 593 tests. FDR threshold of 0.05 considered a significant event. Scale refers to log10 (frequency of P/LP variants in specified genes in each tumor type). Blue rectangles specify significant corrected P values in comparison to non-TCGA ExAC database. ACT adrenocortical carcinoma, CNS central nervous system, EWS Ewing sarcoma, GCT germ cell tumor, HGG high-grade glioma, LGG low-grade glioma, NBL neuroblastoma, OS osteosarcoma, RB retinoblastoma, RHB rhabdomyosarcoma, STS soft tissue sarcoma, WLM Wilms tumor.
Advertisement
“Our findings emphasize the necessity for all C-AYA patients with solid tumors to be sent for genetics evaluation and gene testing,” said Dr. Eng. “Adult guidelines, particularly family history, are typically used to recognize C-AYA patients with possible heritable cancer, but studies have found a family history of cancer in only about 40% of patients with pathogenic and/or likely pathogenic variants.”
The researchers also conducted a drug-target network analysis to determine if the pathogenic and/or likely pathogenic germline variants detected in the dataset were located within genes that could potentially be targeted by drug therapies. Their analysis found that 511 (34%) patients had at least one pathogenic and/or likely pathogenic variant on a gene that is potentially druggable. Notably, they discovered that approximately one-third of these patients had variants that can be targeted by existing FDA-approved drugs.
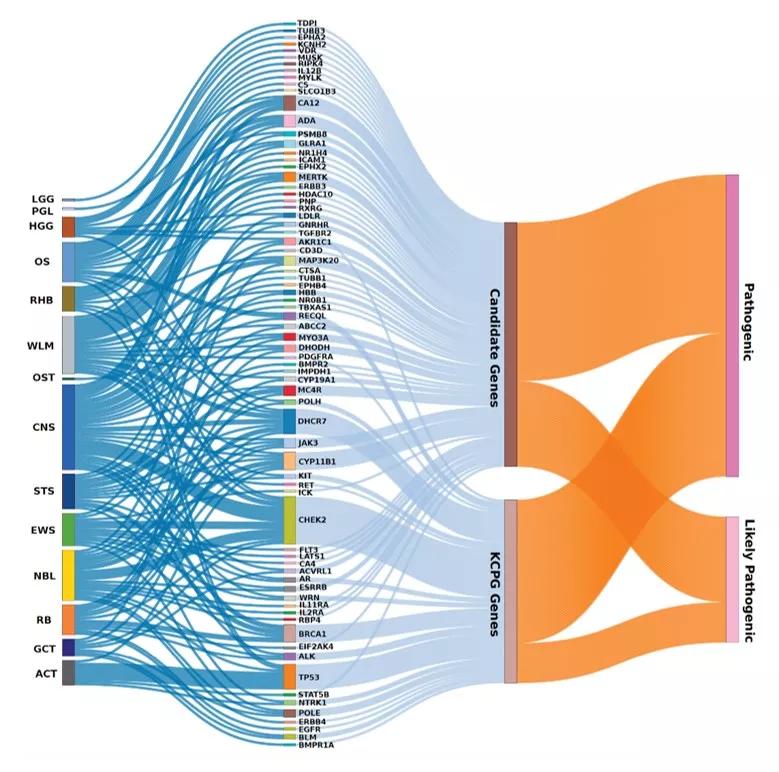
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/7e43ed03-d85b-4b34-97d8-116c2b3fddb7/fig-5-for-web_jpg)
Known cancer-predisposing genes (KCPG) and candidate genes with germline pathogenic/likely pathogenic (P/LP) variants in C-AYA patients with solid tumors that have existing FDA-approved antineoplastic and immunomodulating-related compounds, in regard to their affected tumor types. ACT adrenocortical carcinoma, CNS central nervous system, EWS Ewing sarcoma, GCT germ cell tumor, HGG high-grade glioma, LGG low-grade glioma, NBL neuroblastoma, NM non-malignant tumor, OS osteosarcoma, OST other solid tumors, PGL paraganglioma, RB retinoblastoma, RHB rhabdomyosarcoma, STS soft tissue sarcoma, WLM Wilms tumor.
Advertisement
“Currently, the majority of available targeted therapies are geared to adult patients, leaving few safe and effective treatment options for C-AYA patients,” noted Dr. Eng. “However, we found that a significant number of the germline altered genes in C-AYA solid tumor cancers are targetable by FDA-approved drugs, which presents an opportunity to harness drug repurposing to identify therapeutic options for C-AYA patients.”
Dr. Eng is the inaugural chair of the Genomic Medicine Institute and director of the Center for Personalized Genetic Healthcare. She holds the Sondra J. and Stephen R. Hardis Endowed Chair in Cancer Genomic Medicine.
This work was supported by a VeloSano Pilot Award, belonging to a grant program that provides seed funding for cancer research activities being performed anywhere at Cleveland Clinic.
This article was originally published here.
Advertisement
Advertisement

First full characterization of kidney microbiome unlocks potential to prevent kidney stones

Researchers identify potential path to retaining chemo sensitivity

Large-scale joint study links elevated TMAO blood levels and chronic kidney disease risk over time

Investigators are developing a deep learning model to predict health outcomes in ICUs.

Preclinical work promises large-scale data with minimal bias to inform development of clinical tests

Cleveland Clinic researchers pursue answers on basic science and clinical fronts

Study suggests sex-specific pathways show potential for sex-specific therapeutic approaches

Cleveland Clinic launches Quantum Innovation Catalyzer Program to help start-up companies access advanced research technology