Dose-escalation trial shows local control with low toxicity, low leptomeningeal recurrence
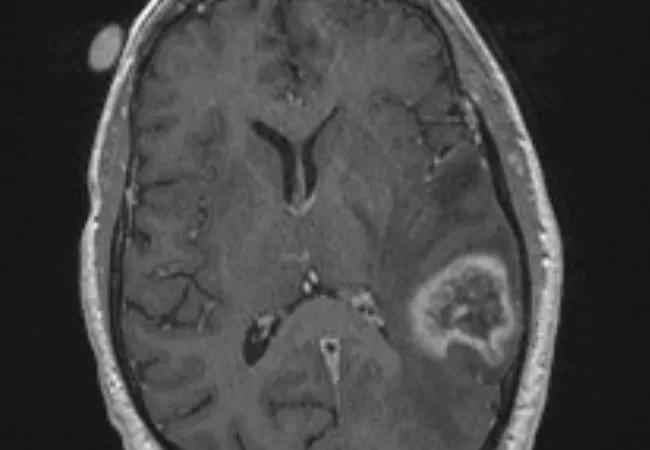
Preoperative (neoadjuvant) stereotactic radiosurgery (SRS) with dose escalation followed by surgical resection for brain metastases > 2 cm yielded local control comparable to that with postoperative SRS or whole-brain radiation therapy (WBRT) — with the benefits of acceptable acute toxicity and a low incidence of leptomeningeal disease.
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
These preliminary findings from a Cleveland Clinic prospective phase 1/2 clinical trial were presented Sept. 15 at the 2019 annual meeting of the American Society for Radiation Oncology (ASTRO).
“So far we have seen excellent results with radiation doses higher than the current SRS standards,” says lead investigator Erin Murphy, MD, a radiation oncologist in Cleveland Clinic’s Rose Ella Burkhardt Brain Tumor and Neuro-Oncology Center. “If findings continue to be positive, we anticipate that new standards can be adopted to safely save more patients from recurring disease.”
For brain metastases larger than 2 cm, Dr. Murphy explains, single-session SRS alone results in local control of only about half the lesions, making surgery an important part of the therapeutic strategy. While WBRT following surgery is effective against recurrence, its use can be associated with significant acute and late toxicity, including a high rate of cognitive decline. Surgical resection followed by SRS to the resection cavity has its own disadvantages, including a significant risk that patients will develop leptomeningeal disease.
Preoperative SRS has been found to offer a number of advantages. Targeting metastases is technically easier before they have been removed, obviating the need to radiate a margin of normal tissue and thereby potentially reducing toxicity. Additionally, systemic chemotherapy can be started earlier, and the risks of leptomeningeal disease and cancer cell spillage may be lower because the tumor margin is “sterilized” preoperatively.
Advertisement
Initial experience with neoadjuvant SRS reported by other investigators (Int J Radiat Oncol Biol Phys. 2014;88:899-906) has been promising. Using a median radiation dose of 14 Gy (range, 11.6-18 Gy) — a 20% reduction from the Radiation Therapy Oncology Group (RTOG) standard — on 47 patients, these researchers reported a good safety profile, but larger lesions were still more likely to recur.
The Cleveland Clinic study is the first to use a dose-escalation strategy starting with the RTOG standard for each tumor size. Two phases are being conducted consecutively:
A total of 27 adults have been enrolled thus far. Their neoadjuvant SRS doses are detailed in the table below.
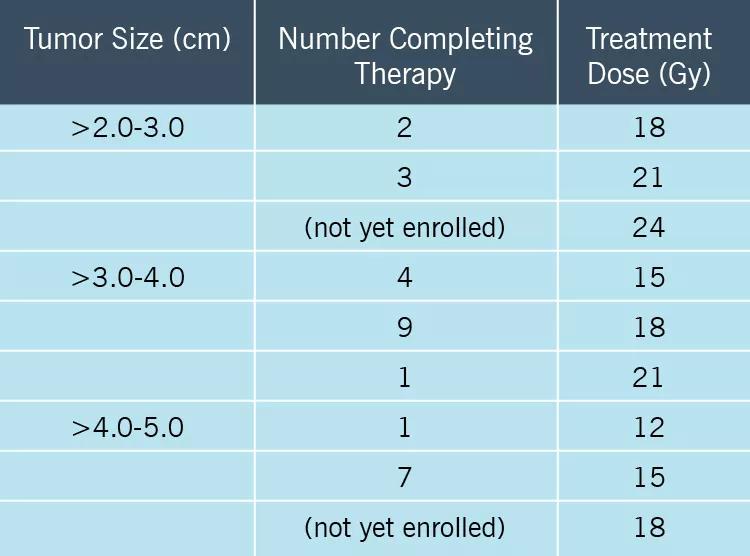
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/8df8afb4-863e-4ecb-b640-8cfedab465e2/19-NEU-3925_InsetTable_jpg)
At mean follow-up of 13.1 months, outcomes were as follows:
Advertisement
The study is continuing to accrue patients for the phase 1 component to determine the maximum tolerated SRS dose levels. Patients will be followed for up to three years to assess for survival, recurrence, radiation necrosis and other possible late effects of neoadjuvant SRS followed by surgical resection. The researchers expect to publish preliminary results next year.
Dr. Murphy says she would like to see the team’s findings replicated in a larger multicenter trial. She anticipates that if results continue to demonstrate safety and efficacy of dose-escalated neoadjuvant SRS, it will become the new standard of care for treating patients with large brain metastases.
“Patients with cancer are living longer thanks to immunotherapy and targeted therapies,” she adds. “It makes sense to use an aggressive local control approach for their brain metastases.”
Advertisement
Advertisement

Combining advanced imaging with targeted therapy in prostate cancer and neuroendocrine tumors

Early results show strong clinical benefit rates

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors