Overall survival extended by approximately 12 months
Advertisement
Cleveland Clinic is a non-profit academic medical center. Advertising on our site helps support our mission. We do not endorse non-Cleveland Clinic products or services. Policy
Although rare, ovarian cancer remains one of the leading causes of cancer-related death in the United States. The American Cancer Society anticipates that in 2018 more than 22,000 women will be diagnosed and 14,000 will die from ovarian cancer. Despite recent advances in medical and surgical techniques used to treat ovarian cancer, mortality remains high because the majority of women are identified at an advanced stage.
Because of ongoing treatment challenges, we are particularly encouraged by a recent trial that demonstrates extended overall survival for patients with newly diagnosed advanced ovarian cancer with the use of hyperthermic intraperitoneal chemotherapy (HIPEC).
Ideal treatment for advanced ovarian cancer includes complete or optimal cytoreductive surgery and platinum/taxane-based chemotherapy. Progression-free survival (PFS) and overall survival (OS) are directly linked to the amount of residual disease following surgery, with optimal surgery defined as < 1 cm residual tumor. Outcomes improve when patients have no gross residual disease following surgery.
There has been and continues to be a debate in gynecologic oncology regarding surgical timing – whether it should take place at presentation or after three cycles of neoadjuvant chemotherapy. A randomized trial conducted in Europe showed that cancer-related outcomes were equivalent. However, surgical morbidity was substantially higher in women undergoing upfront surgery. The trial has been criticized for a number of reasons, leaving many experts still favoring upfront surgery. Nonetheless, we are seeing an increase in the use of neoadjuvant chemotherapy.
Advertisement
Using HIPEC to treat ovarian cancer has been explored at a number of centers around the world. Initially developed to treat rare chemoresistant gastrointestinal malignancies, HIPEC takes place at the time of surgery, once resection is complete. Tubing is placed in the abdomen and chemotherapy is circulated at 42° C. After 45 to 90 minutes, the tubing is removed and the incision closed.
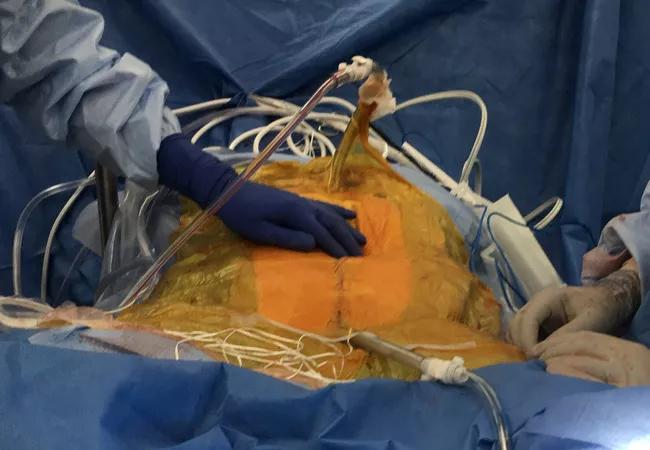
Image content: This image is available to view online.
View image online (https://assets.clevelandclinic.org/transform/120c5e64-2901-4691-a88f-f1ee6879ae94/DeBernardo_18-OBG-198_650x450_jpg)
HIPEC perfusion: Following surgical removal of all gross cancer, tubing is placed in the abdomen and the patient is closed. An assistant at the bedside gently shakes the abdomen and the heated chemotherapy continually circulates for the prescribed time period.
Much retrospective data, and more recently randomized data, suggest there is a benefit in using HIPEC to treat women with recurrent ovarian cancer. A group from the Netherlands reported on a phase 3 randomized trial, published in the New England Journal of Medicine, in which women with newly diagnosed stage III ovarian cancer received HIPEC at interval surgery following neoadjuvant chemotherapy.
Their results demonstrate a significant advantage for women receiving HIPEC, with a hazard ratio of recurrence or death of 0.66 (P = 0.003), PFS of 10.7 versus 14.2 months, and OS of 33.9 versus 45.7 months. Adverse events between the two groups were equivalent.
These data are significant because this is the first time since Gynecologic Oncology Group (GOG)-172, a randomized trial showing the benefit of intraperitoneal chemotherapy over conventional intravenous chemotherapy, that both PFS and OS were improved in women with newly diagnosed ovarian cancer.
Advertisement
As a result of the NEJM study in particular, we are witnessing a number of HIPEC programs opening across the country. Our hope is that these programs offer the clinical expertise required to successfully perform cytoreductive surgery and safely administer HIPEC.
HIPEC is only effective in patients who have minimal or no residual disease after surgery. The radical surgery necessary to achieve these results is generally more successful at high-volume centers with a well-established program and proven track record.
The Cleveland Clinic Ob/Gyn & Women’s Health Institute team managed 144 ovarian cancer surgical cases in 2017, with outcomes above the national average. In addition, we have offered HIPEC to select women with advanced and recurrent ovarian cancer for four years. With well over 100 patients treated, ours is one of the largest programs in the country. We have recently seen a surge in referrals specifically for HIPEC as a result of this new and promising data.
Gynecologic oncologist Dr. DeBernardo established Ob/Gyn & Women’s Health Institute’s HIPEC program and has led it for four years. He is also Director of Minimally Invasive Surgery for the institute.
Advertisement
Advertisement

The shifting role of cell therapy and steroids in the relapsed/refractory setting

Radiation therapy helped shrink hand nodules and improve functionality

Standard of care is linked to better outcomes, but disease recurrence and other risk factors often drive alternative approaches

Phase 1 study demonstrates immune response in three quarters of patients with triple-negative breast cancer

Multidisciplinary teams bring pathological and clinical expertise

Genetic variants exist irrespective of family history or other contributing factors

Study shows significantly reduced risk of mortality and disease complications in patients receiving GLP-1 agonists

Structured interventions enhance sleep, safety and caregiver resiliency in high-acuity units